- Research article
- Open access
- Published: 11 January 2013

The effectiveness of individual interpersonal psychotherapy as a treatment for major depressive disorder in adult outpatients: a systematic review
- Madelon L J M van Hees 1 ,
- Thomas Rotter 1 , 2 ,
- Tim Ellermann 3 &
- Silvia M A A Evers 1 , 4
BMC Psychiatry volume 13 , Article number: 22 ( 2013 ) Cite this article
29k Accesses
40 Citations
54 Altmetric
Metrics details
This systematic review describes a comparison between several standard treatments for major depressive disorder (MDD) in adult outpatients, with a focus on interpersonal psychotherapy (IPT).
Systematic searches of PubMed and PsycINFO studies between January 1970 and August 2012 were performed to identify (C-)RCTs, in which MDD was a primary diagnosis in adult outpatients receiving individual IPT as a monotherapy compared to other forms of psychotherapy and/or pharmacotherapy.
1233 patients were included in eight eligible studies, out of which 854 completed treatment in outpatient facilities. IPT combined with nefazodone improved depressive symptoms significantly better than sole nefazodone, while undefined pharmacotherapy combined with clinical management improved symptoms better than sole IPT. IPT or imipramine hydrochloride with clinical management showed a better outcome than placebo with clinical management. Depressive symptoms were reduced more in CBASP (cognitive behavioral analysis system of psychotherapy) patients in comparison with IPT patients, while IPT reduced symptoms better than usual care and wait list condition.
Conclusions
The differences between treatment effects are very small and often they are not significant. Psychotherapeutic treatments such as IPT and CBT, and/or pharmacotherapy are recommended as first-line treatments for depressed adult outpatients, without favoring one of them, although the individual preferences of patients should be taken into consideration in choosing a treatment.
Peer Review reports
Major depressive disorder (MDD) is a mental disorder characterized by a depressed mood, diminished interest or pleasure, sleeping problems and tiredness, and negative thoughts [ 1 ]. The mean one-year-prevalence of depression in European inhabitants between 18 and 65 years old is 6.9% [ 2 ], and 16.2-16.6% of US adults develop a major depressive disorder [ 3 , 4 ]. Furthermore, depression causes a high burden worldwide, taking fourth place in a ranking of leading contributors to the burden of diseases in 2000. In 2020, it is estimated that depression will take second place in the ranking for all ages and sexes [ 5 ]. Moreover, depression is the leading cause of years of life lived with disability, in all ages and sexes, accounting for 11.9% of all disability [ 6 ]. Since it appears that persons suffering from mental disorders make more use of health care services [ 7 ], the increasing prevalence of depression leads to an increase in health care costs.
Research [ 8 ] and Dutch guidelines [ 9 ] suggest treating depression with psychotherapy and/or medication. Psychotherapy follows several kinds of methodologies. For depression, Cognitive (Behavior) Therapy (CBT) and Interpersonal Psychotherapy (IPT) are often applied. CBT originates from behavior therapy and cognitive therapy, and combines elements of both therapies [ 10 – 12 ]. IPT was originally developed for treating acute depression by improving the interpersonal functioning with important others [ 13 – 17 ]. This study will focus on the effectiveness and efficacy of IPT, since CBT has been subject of many studies up until now, while IPT has only recently become a subject of interest.
As a monotherapy for adults, individual IPT appears to be an effective treatment for depression [ 18 – 20 ], and several reviews [ 21 – 25 ], and meta-analyses [ 26 – 33 ] have been performed on the effectiveness of all kinds of methodologies of psychotherapy. Nevertheless, psychotherapy is a broad concept, and reviews and meta-analyses have often focused on different combinations of psychotherapy for treating depression without comparing one specific sole treatment to another [ 21 , 25 – 30 , 32 , 34 ]. Furthermore, although sole individual IPT appears to be effective, few reviews focus on sole individual IPT in adults with MDD as a primary diagnosis. Often, dissimilar study populations are compared with each other, for example adult, adolescent, and elderly patients in one study [ 23 , 25 – 30 , 33 – 35 ]. Furthermore, several more types of depression exist: dysthymic disorder or depression with medical conditions, for example, but this review will focus only on MDD. Chronic MDD and postpartum depression (PPD) will be included in this systematic review, for the following reasons. First of all, treatment for patients with chronic and non-chronic depression is equal in terms of content and structure. Therefore, the treatments of these patient groups are comparable. Secondly, the symptoms of both kinds of depression are comparable in terms of severity and content, which makes the patients comparable. Furthermore, women with PPD experience the same kind of symptoms as patients with MDD.
Since comorbidity is very common in patients suffering from depression, and this possibly increases the severity of the depression [ 36 – 44 ], this review will focus on MDD as a primary diagnosis with possible comorbidity.
Other factors influencing the results of previously executed systematic reviews include different age groups, in which form the provided IPT is administered, distinct settings, and the time periods during which the studies were executed. IPT is often adjusted for applicability to elderly [ 45 ] or adolescent [ 46 ] depressed patients, or in the form of group IPT [ 47 ]. Therefore, these kinds of treatments may be hard to compare with each other. That is why this review focuses on individual IPT. From here on, when IPT is described in the review, individual IPT is meant, unless described otherwise. Furthermore, the setting in which treatment takes place suggests the depression’s level of severity. It is assumed that inpatients have a more severe depression, which is harder to treat. In addition, inpatient care is often multidisciplinary, which makes it difficult to examine the effects of separate therapies. Research has been conducted on IPT since the 70s, which is why the date limit for this review is set on 1970. This review will give an overview of studies published between January 1970 and August 2012, with a focus on sole IPT administered to adults. Since some therapies have an effect relatively quickly, we did not apply a minimum for duration of a therapy.
With all of the above in mind, the aim of this study is to give an overview of recent literature describing the effectiveness and efficacy of sole individual IPT in comparison with standardized forms of treatment for treating patients with MDD as a primary diagnosis. The following research question has been formulated: Is individual interpersonal psychotherapy more preferable in comparison with other standardized forms of treatment for treating adult outpatients with a primary diagnosis of major depressive disorder?
In order to answer this question, a systematic review will be performed on RCTs and C-RCTs comparing the effectiveness (the outcome of a new treatment compared to other kinds of treatment(s), usually in a clinical setting) or efficacy (the outcome of treatments in homogeneous patient groups, usually in an experimental setting) [ 48 ] of individual sole IPT with other standardized forms of treatment, for treating adult outpatients with MDD as a primary diagnosis.
This paragraph will outline which steps were taken in order to perform this systematic review. An overview of the methods used for data collection, study selection, and data analysis will be provided.
Data sources
RCTs about IPT for depression were collected by searching PubMed and PsycINFO for studies published between January 1970 and August 2012. The following medical subject heading (MeSH) categories and keywords were used: depression, postpartum depression, major depressive disorder, dysthymic disorder, interpersonal psychotherapy, treatment outcome, clinical trials. The exact search terms and MeSH headings can be found in the additional files (Additional file 1 – Search strategy). All titles and abstracts were screened, and only studies which met the review inclusion criteria (see next paragraph and Table 1 ) were selected for further review. Citation tracking and snowballing techniques added studies to the second screening phase, in which selected studies were screened for eligibility using a predefined checklist (see Data analysis) (Additional file 2 – Checklist).
Study selection
Only studies with sufficient methodological quality meeting the inclusion criteria were selected for this review. The criteria for selection will be described shortly. An overview of the inclusion and exclusion criteria is provided in Table 1 .
Studies were included if they were randomized or cluster-randomized evaluations (RCTs or C-RCTs) published in English after January 1 st , 1970, and took place in western jurisdictions, to ensure high internal validity. These studies had to focus on MDD (non-chronic or chronic) as a primary diagnosis in adults (18–65 years old). The diagnosis must have been reached using a formal classification system, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) [ 1 ], the International Classification of Diseases (ICD) [ 49 ], or the Research Diagnostic Criteria [ 50 ]. Bipolar disorders as primary diagnoses were excluded, as well as cases where the patients were elderly people or adolescents, or in cases in which physical conditions might contribute to the (severity of) depressive symptoms. The proposed intervention must have been individual sole IPT, in comparison with other psychotherapies, pharmacotherapy, or combined treatment. Group IPT and other kinds of treatments were excluded. Studies executed in ambulant care or primary care were included, whereas inpatient care patients were excluded.
By making the inclusion criteria very strict, a more homogeneous group, with a narrower scope, was created, which made it possible to focus on clinical applicability of the treatments for these kinds of patients.
Data analysis
Before the data were analyzed for this review, the methodological quality of the studies included after screening has been assessed, using a predefined checklist (Additional file 2 – Checklist). This checklist was composed of Delphi-list questions [ 51 ] and questions assessing the risk of bias in effect evaluation studies [ 52 ]. General questions were composed for collecting relevant information about the study, after which the resulting information was entered in a Microsoft Excel table for a clear overview. This overview was used to create a table of evidence of the extracted study data, and to summarize the most important findings. MH performed the analysis and consulted TR in case of doubt. In this case, the analyses were double checked and consensus was reached.
The literature search resulted in 3981 studies, of which 3911 were excluded from further review for several reasons, documented below. Figure 1 shows the flow diagram of included and excluded studies. Studies were excluded when they did not meet the inclusion criteria, based on the title and abstract: i.e. they did not focus on MDD as a primary diagnosis, on individual sole IPT, or the target group was anything other than adults. Another 62 were excluded after reading the full text, leaving 8 articles eligible for this review.
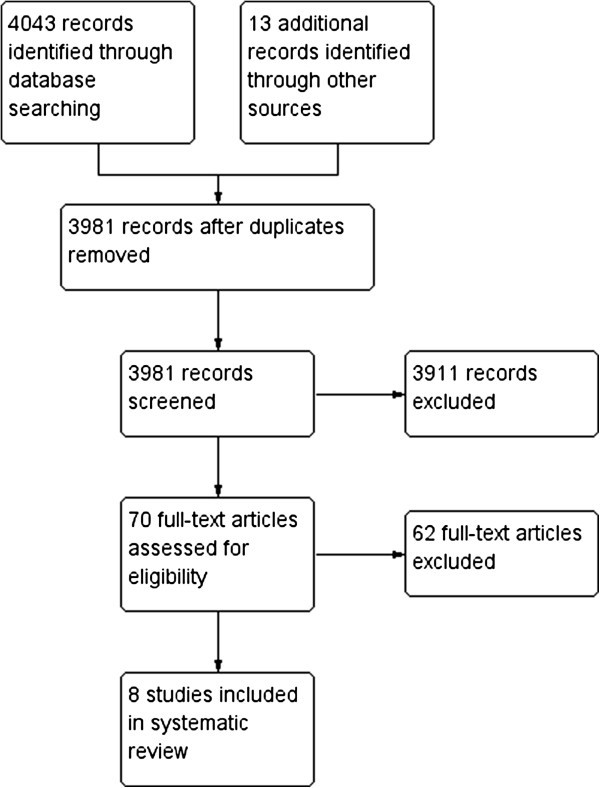
Flow diagram of included and excluded articles; reasons in Additional file 3 .
These 62 full-text articles were excluded for the following reasons: being reviews or meta-analyses [ 21 – 23 , 25 – 30 , 32 , 34 , 53 – 60 ], being a protocol for a study [ 61 ], being a study based on earlier/other studies [ 43 , 45 , 62 – 76 ], there was no comparison in the study [ 77 ], MDD was not the primary diagnosis [ 78 – 82 ], the study had the wrong aim for this review [ 83 – 86 ], there was no research data [ 87 – 91 ], or one of the interventions was not IPT as described in the eligibility criteria [ 35 , 92 – 100 ]. See Additional file 3 – List of excluded studies for a detailed description of the reasons for exclusion.
Description of the studies
The main characteristics of the RCT studies included are summarized in Table 2 . One study was carried out in the Netherlands [ 101 ], one in New Zealand [ 102 ], one in Canada [ 103 ], one in the UK [ 104 ], one in Germany [ 105 ], and three in the USA [ 106 – 108 ]. All studies clearly described eligibility criteria and success-of-treatment point. All but two [ 103 , 104 ] included an intention-to-treat analysis. Seven studies reported comparable sociodemographic and psychiatric variables at baseline. One [ 103 ] did not report these variables.
A total of 1233 patients were included in the review, of whom 854 completed treatment in outpatient facilities. Of the patients included, 392 received IPT, 14 received CBASP (Cognitive Behavioral Analysis System of Psychotherapy), 160 received CBT, 153 received pharmacotherapy (nefazodone, nortriptyline hydrochloride, or venlafaxine hydrochloride), 67 received pharmacotherapy plus clinical management, 49 received IPT and nefazodone, 47 received IPT and a placebo, 34 received a placebo plus clinical management, 92 received usual care consisting of communication with a physician for appropriate treatment, and 51 were put on a wait list. The mean age in seven studies [ 101 , 102 , 104 – 106 ] ranged from 29.4 to 40.2 years old, and the percentage of female patients varied from 55% to 83%, except for one study, in which only females participated [ 108 ]. One study did not report these data [ 103 ]. All patients were diagnosed with non-psychotic MDD as a primary diagnosis according to the DSM-III-R [ 109 ], DSM-IV [ 110 ], or the Research Diagnostic Criteria [ 50 ].
IPT in all studies was based on a standardized manual [ 14 , 17 ], as was CBASP [ 111 ] and CBT [ 12 , 112 , 113 ]. The number of IPT and CBT sessions varied from 8 to 24 in a 12- or 16-week period, and most of the sessions were held weekly. Physicians administering nefazodone or nortriptyline were instructed to follow a manual. Patients receiving nefazodone started at 100 mg capsules per day, and doses were gradually increased to a minimum of 400 mg, with a maximum of 600 mg [ 101 ]. Patients receiving nortriptyline started at 25 mg per day, aiming for blood levels of 190–570 nmol/liter [ 106 ]. Patients receiving imipramine hydrochloride had a dosage between 150 and 185 mg. Pharmacotherapists administering venlafaxine followed an evidence-based protocol of 37.5 mg twice-daily doses [ 104 ]. Pharmacotherapy plus clinical management was administered by a psychiatrist who followed the client for the duration of the protocol associated with the antidepressant medication [ 103 ], or as long as the clinical management would be administered [ 107 ].
Risk of bias
Risk of bias was measured and summarized (see Figure 2 ) according to the standards of the Cochrane Collaboration [ 52 ]. Although this was not always described exhaustively, all studies used randomization and seemed to present complete outcome data. Therefore, all included studies had a low risk of selection bias and attrition bias. Nevertheless, two studies [ 103 , 107 ] had an unclear risk of detection bias and one of them [ 103 ] had a high risk of reporting bias. Another study [ 108 ] had a high risk of detection bias. Notwithstanding these higher levels of bias, these studies have been included in this review.
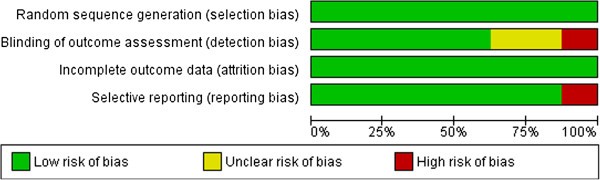
Summary of risk of bias in six studies.
Findings on outcome measurements
The outcome of the HAMD showed an overall decrease in the level of depression over time ( p <0.001) between the four treatment conditions (IPT, nefazodone, IPT and nefazodone, IPT and placebo), but this was not statistically significant. A significant difference was found between IPT and nefazodone and the use of nefazodone without IPT in favor of the first (for the intent to treat sample: adjusted OR (95% CI)=3.22 (1.02-10.12), p =0.045). Furthermore, a significant difference was found in the MADRS scores. Patients receiving IPT with nefazodone improved more than did patients receiving nefazodone without IPT. Furthermore, the nefazodone condition showed only a small improvement after the first six weeks [ 101 ].
Imipramine hydrochloride combined with clinical management (CM) was significantly superior to placebo with CM on general level of functioning. Patients receiving IPT or imipramine hydrochloride with CM appeared to have a better outcome on the HRSD than patients receiving placebo with CM ( p =0.018 and p =0.017). Furthermore, these patients showed a significantly higher percentage in the recovery analysis compared to placebo with CM patients, measured by a score of six or lower on the HRSD ( p =0.010 and p =0.013) [ 107 ].
In the Luty et al. study [ 102 ], depressive symptoms improved for about 55%. No statistically significant differences were found between IPT and CBT on the primary outcome measure MADRS (9.5% mean difference (95% CI), p =0.059), nor after controlling for baseline severity ( p =0.055).
HRSD scores were significantly higher in the IPT condition compared to the PHT-CM condition ( t (96)=−2.46, p <0.05, d =−0.50). No significant differences were found between IPT and CBT conditions ( t (96)=−1.19, p =0.46, d =−0.24), or between CBT and PHT-CM conditions ( t (96)=−1.35, p =0.37, d =−0.28) [ 103 ].
Depressive symptoms, measured by the HAMD and BDI, improved significantly ( p <0.001) in the first six weeks for patients receiving IPT or venlafaxine [ 104 ]. Although the venlafaxine group showed a slightly better outcome than the IPT group, no significant differences were found after six weeks.
O’Hara described recovery rates for women with PPD based on HRSD scores and BDI scores, favoring IPT over wait list condition (WLC). Based on HRSD scores (HRSD ≤6), IPT had a recovery rate of 31.7%, compared to 15% of WLC ( p =0.03). Based on BDI scores (BDI ≤9), IPT had a recovery rate of 38.3%, while women in the WLC group showed a recovery rate of 18.3% ( p =0.02) [ 108 ].
In both the IPT and CBASP group [ 105 ], HRSD scores decreased after 16 weeks, but only in the CBASP group statistical significance was reached ( t (13)=3.53, p =0.004). BDI scores were significantly lower after 16 weeks in both groups (IPT: t (14)=2.34, p =0.034; CBASP: t (13)=5.01, p <0.001). HRSD scores did not show a significant difference between the groups, whereas BDI scores showed a significantly higher reduction in depressive symptoms in the CBASP group after 16 weeks (mean BDI score of 10.79 vs. 21.27 in IPT; F (1,26)=4.34, p =0.047, treatment effect size: Cohen’s d =0.87).
Eight months after the start of the treatments (IPT, nortriptyline, or usual care), all HRSD scores improved significantly (χ 2 =816.14, df =6, p <0.001), and a significant difference was found between the groups (χ 2 =14.92, df =2, p =0.001). Post-hoc group t -test comparisons showed significant differences ( p <0.01) in HRSD scores between nortriptyline and usual care, at most measurement times favoring nortriptyline, and between IPT and usual care, favoring IPT after eight months. No significant difference was found between IPT and nortriptyline at any moment in time [ 106 ].
Meta-analysis and summary of findings
As can be seen in Table 2 , heterogeneity between the studies exists, which made it difficult to make meta-analytic comparisons. However, three studies were comparable in terms of measuring the effects of IPT and CBT [ 102 , 103 , 107 ]. The mean difference between the treatments was 1.01 (95% CI: -0.34, 2.37) favoring CBT over IPT, but did not reach a statistically significant level. See Figure 3 for more detailed information.
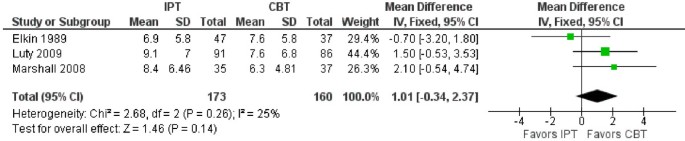
Comparison of HRSD scores between IPT and CBT. CBT Cognitive Behavior Therapy; IPT Interpersonal Psychotherapy; SD Standard Deviation.
Although no further meta-analyses were possible, and results appeared to be inconsistent, some conclusions can be drawn from these studies. IPT combined with nefazodone improved MADRS scores significantly better than did nefazodone alone [ 101 ]. Furthermore, higher HRSD scores were found in IPT patients in comparison with PHT-CM patients [ 103 ]. IPT patients showed a significantly greater decrease of HRSD and BDI scores than WLC patients [ 108 ]. As measured with the BDI, depressive symptoms were reduced more in CBASP patients in comparison with IPT patients [ 105 ]. Finally, IPT patients produced lower HRSD scores in comparison with patients receiving usual care [ 106 ].
Main results
The results of this systematic review show inconsistent findings in the eight heterogeneous studies included. The effectiveness and efficacy of the several treatments is comparable in most studies, and some conclusions may be drawn. Overall, the efficacy of IPT and CBT appears to be equal [ 102 ]. Contradictory results were found in IPT in comparison with pharmacotherapy. IPT combined with nefazodone appears to have a higher efficacy than sole nefazodone [ 101 ], while pharmacotherapy combined with clinical management appears to have a higher efficacy than IPT alone [ 103 ]. However, another study showed comparable results between IPT and imipramine hydrochloride with clinical management (CM), which both returned a better outcome on the HRSD compared to placebo with CM [ 107 ]. Furthermore, venlafaxine seems to reduce depressive symptoms more than IPT after six weeks, although this outcome was not significant [ 104 ]. The effects of using sole IPT and sole nortriptyline do not significantly differ from each other [ 106 ]. IPT and CBASP appear to be very comparable in efficacy, although scores of the BDI showed a slight preference for CBASP [ 105 ]. Finally, IPT appears to be more effective than wait list condition [ 108 ], and usual care after eight months, as does nortriptyline [ 106 ].
These outcomes suggest that several kinds of treatments are effective or efficacious for depressed patients, although one has to keep in mind the small number of included studies. Patients are recommended to choose a treatment which fits their personal preferences, since this may affect the outcome of the treatment. Policy makers are advised to base regulations on the effectiveness and efficacy of treatments in general, instead of a slightly different effect between one treatment and the other, since these studies do not take individual differences and preferences into account.
Limitations
This review has a number of limitations. First, this review included only adult outpatients with unipolar, non-psychotic major depression as a primary diagnosis. Although these inclusion criteria were a deliberate choice, this review has consequences for the generalizability. These results are not generalizable to children, adolescents, or the elderly, to patients with other kinds of depression, or to patients suffering from a combination of depression and medical conditions, or from depression and substance abuse. Furthermore, no distinction has been made in the severity of depression, which causes a higher heterogeneity in the complete sample, making results more uncertain.
Second, only eight studies with a limited number of participants were included in this review. Although most studies showed a low risk of bias, the small size of the sample may increase this risk. Furthermore, results are harder to generalize with a small number of participants, especially because many different kinds of treatments have been compared with each other (high heterogeneity), which limited the number of participants in the groups not receiving IPT. Moreover, the limited number of included studies in this review, makes one question the applicability of the Cochrane guidelines for conducting a systematic review [ 52 ], for clinical treatments in mental health care.
Third, all included patients were outpatients and therefore had to be willing and motivated to participate in the selected studies. This may cause some bias, since not all types of patients could be included in the studies. For example, treatment-resistant depressed patients may have been less motivated than patients who were not treatment-resistant, and it may not be possible to generalize results for these patients.
Fourth, pharmacotherapy consisted of different types of antidepressant medication. Although these medications may seem to be equally effective, some differences may exist, which may interfere with the results of this review. Furthermore, one study [ 101 ] used nefazodone as pharmacotherapy, although this medication has been withdrawn in, amongst other countries, the USA and the Netherlands, because of hepatotoxicity associated with this drug [ 114 ].
Fifth, some of the findings were based on the scores of the HRSD [ 101 , 103 – 108 ]. However, this scale has recently been criticized for having multiple problems, including among others the existence of different versions and not being as sensitive as other scales [ 115 , 116 ]. Despite these flaws, the HRSD has been used in many studies and the outcomes of this scale can therefore not be excluded from this review. Furthermore, findings were also based on the MADRS [ 101 , 102 ], which is more sensitive to treatment effect than the HAMD [ 117 ], and on the BDI [ 104 , 105 ] which correlates weakly with the HDRS [ 118 ] and has several advantages and disadvantages [ 119 ], but is widely used.
Sixth, one study [ 104 ] measured the efficacy only after six weeks, without follow-up measurement. This is a very short period for measuring the efficacy of IPT. Therefore, the results of this study may be questionable. Furthermore, these authors did not include an intention-to-treat analysis, which increases the risk of bias.
Finally, although a profound search has been performed, there is no complete certainty that all studies eligible for this review have been found. Furthermore, the search was directed only at published studies, automatically excluding unpublished data, causing possible publication bias.
It can be concluded that the differences between the effects and efficacy of several types of treatment are very small and they are often not significant. This in turn is consistent with a study concluding that the effects of psychotherapy for adult depression in meta-analyses are overestimated [ 27 ]. Nevertheless, usual care, as described in the study of Schulberg et al. [ 106 ], appears to be ineffective and is not recommended as a treatment for MDD. Therefore, psychotherapeutic treatments such as IPT and CBT, and/or pharmacotherapy are recommended as first-line treatments for depressed adult outpatients. This conclusion is consistent with a previous study [ 21 ], and review [ 26 ], and previous meta-analyses [ 28 , 29 , 32 , 33 , 55 ],although, as has been stated in the introduction, these studies had several limitations as well. Furthermore, it is recommended that the type of treatment is adjusted to the individual preferences of the patient.
Future research should focus on a larger sample including patients with MDD, while correcting for severity of depression. Since many studies focused on IPT combined with medication, it is recommended that these studies be included in future research as well. Furthermore, it is recommended that future studies included in a review, have longer follow-up periods. All studies should aim for the highest quality standards currently set.
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revised. 2000, Washington DC: American Psychiatric Association
Google Scholar
Wittchen HU, Jacobi F: Size and burden of mental disorders in Europe - a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 2005, 15: 357-376. 10.1016/j.euroneuro.2005.04.012.
CAS PubMed Google Scholar
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS: The epidemiology of major depressive disorder. Results from the national comorbidity survey replication (NCS-R). J Am Med Assoc. 2003, 289: 3095-3105. 10.1001/jama.289.23.3095.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE: Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005, 62: 593-602. 10.1001/archpsyc.62.6.593.
PubMed Google Scholar
Depression. http://www.who.int/mental_health/management/depression/definition/en/ .
World Health Organization: The World health report: 2001: Mental health: new understanding, new hope. 2001
Kessler RC, Demler O, Frank RG, Olfsen M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM: US prevalence and treatment of mental disorders: 1990–2003. N Engl J Med. 2005, 352 (24): 2515-2523. 10.1056/NEJMsa043266.
CAS PubMed PubMed Central Google Scholar
Weihs K, Wert JM: A primary care focus on the treatment of patients with major depressive disorder. Am J Med Sci. 2011, XXX: XXX-
Depressie (eerste revisie). Richtlijn voor de diagnostiek, behandeling en begeleiding van volwassen patiënten met een depressieve stoornis. http://www.psychiatrie-nederland.nl/Multidisciplinaire%20Richtlijn%20Depressie%20eerste%20update%202010.pdf .
Boelens W: Protocollaire behandeling van depressieve patiënten: cognitieve gedragstherapie. Protocollaire behandelingen in de ambulante geestelijke gezondheidszorg, deel I. Edited by: Keijsers GPJ, Minnen A, Hoogduin CAL. 2004, Houten: Bohn Stafleu Van Loghum, 154-182.
Lewinsohn PM: The behavioral study and treatment of depression. Progress in behavior modification, volume 1. Edited by: Hersen M, Eisler RM, Miller PM. 1975, New York: Academic Press
Beck AT, Rush AJ, Shaw BF, Emery G: Cognitive Therapy of Depression. 1979, New York: Guilford
Blom MBJ, Jonker K: Protocollaire behandeling van depressieve patiënten: interpersoonlijke psychotherapie. Protocollaire behandelingen in de ambulante geestelijke gezondheidszorg, deel II. Edited by: Keijsers GPJ, Minnen A, Hoogduin CAL. 2004, Houten: Bohn Stafleu Van Loghum, 96-116.
Klerman GL, Weismann MM, Rounsaville BJ, Chevron ES: Interpersonal Psychotherapy of Depression. 1984, New York: Basic Books
Klerman GL, Weissman MM: New applications of interpersonal psychotherapy. 1994, Washington, DC: American Psychiatric Press
Jonker K, Blom M: Interpersoonlijke psychotherapie. Depressie: theorie, diagnostiek en behandeling. Edited by: Albersnagel FA, Emmelkamp PMG, Hoofdakker RH. 1998, Houten/Diegem: Bohn Stafleu Van Loghum, 251-271.
Weissman MM, Markowitz JC, Klerman GL: Comprehensive guide to interpersonal psychotherapy. 2000, New York: Basic Books
DiMascio A, Weissman MM, Prusoff BA, Neu C, Zwilling M, Klerman GL: Differential symptom reduction by drugs and psychotherapy in acute depression. Arch Gen Psychiatry. 1979, 36: 1450-1456. 10.1001/archpsyc.1979.01780130068008.
Weissman MM, Prusoff BA, DiMascio A, Neu C, Goklaney M, Klerman GL: The efficacy of drugs and psychotherapy in the treatment of acute depressive episodes. Am J Psychiatry. 1979, 136: 555-558.
Reynolds CF, Frank E, Perel JM, Imber SD, Cornes C, Morycz RK, Mazumdar S, Miller MD, Pollock BG, Hind Rufai A, et al: Combined pharmacotherapy and psychotherapy in the acute and continuation treatment of elderly patients with recurrent major depression: a prelimenary report. Am J Psychiatry. 1992, 149: 1687-1692.
Cascalenda N, Perry JC, Looper K: Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry. 2002, 159: 1354-1360. 10.1176/appi.ajp.159.8.1354.
De Mello MF, De Jesus Mari J, Bacaltchuk J, Verdeli H, Neugebauer R: A systematic review of research findings of the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005, 255: 75-82. 10.1007/s00406-004-0542-x.
Hollon SD, Ponniah K: A review of empirically supported psychological therapies for mood disorders in adults. Depress Anxiety. 2010, 27: 891-932. 10.1002/da.20741.
PubMed PubMed Central Google Scholar
Parker G, Parker I, Brotchie H, Stuart S: Interpersonal psychotherapy for depression? The need to define its ecological niche. J Affect Disord. 2006, 95: 1-11. 10.1016/j.jad.2006.03.019.
Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C: Combined pharmacotherapy and psychological treatment for depression. A systematic review. Arch Gen Psychiatry. 2004, 61: 714-719. 10.1001/archpsyc.61.7.714.
Cuijpers P, Dekker J, Hollon SD, Andersson G: Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009, 70: 1219-1229. 10.4088/JCP.09r05021.
Cuijpers P, Van Straten A, Bohlmeijer E, Hollon SD, Andersson G: The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol Med. 2010, 40: 211-223. 10.1017/S0033291709006114.
Cuijpers P, Van Straten A, Hollon SD, Andersson G: The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010, 121: 415-423.
Cuijpers P, Van Straten A, Van Oppen P, Andersson G: Are psychological and pharmacologic interventions equally effective in the treatment of adult depressive disorders? A meta-analysis of comparative studies. J Clin Psychiatry. 2008, 69: 1675-1685. 10.4088/JCP.v69n1102.
Cuijpers P, Van Straten A, Van Schaik A, Andersson G: Psychological treatment of depression in primary care: a meta-analysis. Br J Gen Pract. 2009, 59: e51-e60.
Gloaguen V, Cottraux J, Cucherat M, Blackburn IM: A meta-analysis of the effects of cognitive therapy in depressed patients. J Affect Disord. 1998, 49: 59-72. 10.1016/S0165-0327(97)00199-7.
Guidi J, Fava GA, Fava M, Papakostas GI: Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: a preliminary meta-analysis. Psychol Med. 2011, 41: 321-331. 10.1017/S0033291710000826.
Cuijpers P, Geraedts AS, Van Oppen P, Andersson G, Markowitz JC, Van Straten A: Interpersonal psychotherapy for depression: a meta-analysis. Am J Psychiatry. 2011, 168: 581-592. 10.1176/appi.ajp.2010.10101411.
Thase ME, Greenhouse JB, Frank E, Reynolds CF, Pilkonis PA, Hurley K, Grochocinski V, Kupfer DJ: Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997, 54: 1009-1015. 10.1001/archpsyc.1997.01830230043006.
De Mello MF, Myczcowisk LM, Menezes PR: A randomized controlled trial comparing moclobemide and moclobemide plus interpersonal psychotherapy in the treatment of dysthymic disorder. J Psychother Pract Res. 2001, 10: 117-123.
Kroenke K, Shen J, Oxman TE, Williams JW, Dietrich AJ: Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008, 134: 209-215. 10.1016/j.pain.2007.09.021.
Poleshuck EL, Bair MJ, Kroenke K, Watts A, Tu X, Giles DE: Pain and depression in gynecology patients. Psychosomatics. 2009, 50: 270-276. 10.1176/appi.psy.50.3.270.
Poleshuck EL, Talbot NL, Su H, Tu X, Chaudron L, Gamble S, Giles DE: Pain as a predictor of depression treatment outcomes in women with childhood sexual abuse. Compr Psychiatry. 2009, 50: 215-220. 10.1016/j.comppsych.2008.08.001.
Karp JF, Scott J, Houck P, Reynolds CF, Kupfer DJ, Frank E: Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005, 66: 591-597. 10.4088/JCP.v66n0508.
Yates WR, Mitchell J, Rush J, Trivedi MH, Wisniewski SR, Warden D, Hauger RB, Fava M, Gaynes BN, Husain MM, et al: Clinical features of depressed outpatients with and without co-occurring general medical conditions in STAR*D. Gen Hosp Psychiatry. 2004, 26: 421-429. 10.1016/j.genhosppsych.2004.06.008.
Ferrando SJ, Freyberg Z: Treatment of depression in HIV positive individuals: a critical review. Int Rev Psychiatry. 2008, 20: 61-71. 10.1080/09540260701862060.
Andreescu C, Lenze EJ, Dew MA, Begley AE, Mulsant BH, Dombrovski AY, Pollock BG, Stack J, Miller MD, Reynolds CF: Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007, 190: 344-349. 10.1192/bjp.bp.106.027169.
Brown C, Schulberg HC, Madonia MJ, Shear MK, Houck PR: Treatment outcomes for primary care patients with major depression and lifetime anxiety disorders. Am J Psychiatry. 1996, 153: 1293-1300.
Young JF, Mufson L, Davies M: Impact of comorbid anxiety in an effectiveness study of interpersonal psychotherapy for depressed adolescents. J Am Acad Child Adolesc Psychiatry. 2006, 45: 904-912. 10.1097/01.chi.0000222791.23927.5f.
Reynolds CF, Frank E, Kupfer DJ, Thase ME, Perel JM, Mazumdar S, Houck PR: Treatment outcome in recurrent major depression: a post hoc comparison of elderly ("young old") and midlife patients. Am J Psychiatry. 1996, 153: 1288-1292.
Mufson L, Moreau D, Weissman MM, Klerman GL: Interpersonal Therapy for Depressed Adolescents. 1993, New York: Guilford Press
Levkovitz Y, Shahar G, Native G, Hirsfeld E, Treves I, Krieger I, Fennig S: Group interpersonal psychotherapy for patients with major depression disorder - pilot study. J Affect Disord. 2000, 60: 191-195. 10.1016/S0165-0327(99)00181-0.
Lutz W: Efficacy, Effectiveness, and Expected Treatment. Respnse in Psychotherapy. J Clin Psychol. 2003, 59: 745-750. 10.1002/jclp.10169.
World Health Organization: International Statistical Classification of Diseases, 10th Revision (ICD-10). 1992, Geneva: World Health Organization
Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry. 1978, 35: 773-782. 10.1001/archpsyc.1978.01770300115013.
Verhagen AP, De Vet HCW, De Bie RA, Kessels AGH, Boers M, Bouter LM, Knipschild PG: The Delphi-List: a criteria list for quality assessment of randomized controlled trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998, 51: 1235-1241. 10.1016/S0895-4356(98)00131-0.
Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Edited by: Higgins JPT, Green S. 2011, The Cochrane Collaboration, Available from http://www.cochrane-handbook.org .
Weissman MM: Recent non-medication trials of interpersonal psychotherapy for depression. Int J Neuropsychopharmacol. 2007, 10: 117-122. 10.1017/S1461145706006936.
Jakobsen JC, Hansen JL, Simonsen E, Gluud C: The effect of adding psychodynamic therapy to antidepressants in patients with major depressive disorder. A systematic review of randomized clinical trials with meta-analyses and trial sequential analyses. J Affect Disord. 2011, in press
De Maat SM, Dekker J, Schoevers RA, De Jonghe F: Relative efficacy of psychotherapy and combined therapy in the treatment of depression: a meta-analysis. Eur Psychiatry. 2007, 22: 1-8.
Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ: Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment?. J Clin Psychiatry. 2005, 66: 455-468. 10.4088/JCP.v66n0408.
Dorrepaal E, Van Nieuwenhuizen C, Schene A, De Haan R: De effectiviteit van cognitieve en interpersoonlijke therapie bij depressiebehandeling: een meta-analyse. Tijdschr Psychiatr. 1998, 40: 27-39.
Kotova E: A meta-analysis of Interpersonal Psychotherapy, Dissertation Abstracts International: Section B. Sciences and Engineering. 2005, 66 (5-B): 2828-
Cuijpers P, Andersson G, Donker T, van Straten A: Psychological treatment of depression: results of a series of meta-analyses. Nord J Psychiatry. 2011, 65 (6): 354-364. 10.3109/08039488.2011.596570.
Cuijpers P, van Straten A, Schuurmans J, van Oppen P, Hollon SD, Andersson G: Psychotherapy for chronic major depression and dysthymia: A meta-analysis. Clin Psychol Rev. 2010, 30 (1): 51-62. 10.1016/j.cpr.2009.09.003.
Kriston L, Von Wolff A, Hölzel L: Effectiveness of psychotherapeutic, pharmacological, and combined treatments for chronic depression: a systematic review (METACHRON). BMC Psychiatry. 2010, 10: 95-10.1186/1471-244X-10-95.
Blom MBJ, Spinhoven P, Hoffman T, Jonker K, Hoencamp E, Haffmans PMJ, Van Dyck R: Severity and duration of depression, not personality factors, predict short term outcome in the treatment of major depression. J Affect Disord. 2007, 104: 119-126. 10.1016/j.jad.2007.03.010.
Coulehan JL, Schulberg HC, Block MR, Madonia MJ, Rodriguez E: Treating depressed primary care patients improves their physical, mental, and social functioning. Arch Intern Med. 1997, 157: 1113-1120. 10.1001/archinte.1997.00440310079008.
Watkins JT, Leber WR, Imber SD, Collins JF, Elkin I, Pilkonis PA, Sotsky SM, Shea MT, Glass DR: Temporal course of change of depression. J Consult Clin Psychol. 1993, 61: 858-864.
Ablon JS, Jones EE: Validity of controlled clinical trials of psychotherapy: findings from the NIMH treatment of depression collaborative research program. Am J Psychiatry. 2002, 159: 775-783. 10.1176/appi.ajp.159.5.775.
Agosti V, Ocepek-Welikson K: The efficacy of imipramine and psychotherapy in early-onset chronic depression: a reanalysis of the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Affect Disord. 1997, 43: 181-186. 10.1016/S0165-0327(97)01428-6.
Barber JP, Muenz LR: The role of avoidance and obsessiveness in matching patients to cognitive and interpersonal psychotherapy: empirical findings from the Treatment for Depression Collaborative Research Program. J Consult Clin Psychol. 1996, 64: 951-958.
Blom MBJ, Hoek HW, Spinhoven P, Hoencamp E, Haffmans PMJ, Van Dyck R: Treatment of depression in patients from ethnic minority groups in the Netherlands. Transcult Psychiatry. 2010, 47: 473-490. 10.1177/1363461510374561.
Brown C, Schulberg HC, Sacco D, Perel JM, Houck PR: Effectiveness of treatments for major depression in primary medical care practice: a post hoc analysis of outcomes for African American and white patients. J Affect Disord. 1999, 53: 185-192. 10.1016/S0165-0327(98)00120-7.
Carter JD, Luty SE, McKenzie JM, Mulder RT, Frampton CM, Joyce PR: Patient predictors of response to cognitive behaviour therapy and interpersonal psychotherapy in a randomised clinical trial for depression. J Affect Disord. 2011, 128: 252-261. 10.1016/j.jad.2010.07.002.
Elkin I, Gibbons RD, Shea MT, Sotsky SM, Watkins JT, Pilkonis PA, Hedeker D: Initial severity and differential treatment outcome in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 1995, 63: 841-847.
Imber SD, Pilkonis PA, Sotsky SM, Elkin I, Watkins JT, Collins JF, Shea MT, Leber WR, Glass DR: Mode-specific effects among three treatments for depression. J Consult Clin Psychol. 1990, 58: 352-359.
Lave JR, Frank RG, Schulberg HC, Kamlet MS: Cost-effectiveness of treatments for major depression in primary care practice. Arch Gen Psychiatry. 1998, 55: 645-651. 10.1001/archpsyc.55.7.645.
Kim DM: Therapist effects and treatment effects in psychotherapy: Analysis on the National Institute of Mental Health Treatment of Depression Collaborative Research Program (NIMH TDCRP). 2003, Wisconsin: ProQuest Information & Learning, Dissertation Abstracts International: Section B: The Sciences and Engineering
Shea MT, Elkin I, Imber SD, Sotsky SM, Watkins JT, Collins JF, Pilkonis PA, Beckham E, Glass DR, Dolan RT, et al: Course of depressive symptoms over follow-up. Findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry. 1992, 49 (10): 782-787. 10.1001/archpsyc.1992.01820100026006.
Segal ZV, Whitney DK, Lam RW, Group CDW: Clinical guidelines for the treatment of depressive disorders. III. Psychotherapy. Can J Psychiatry. 2001, 46 (Suppl 1): 29S-37S.
Blom MBJ, Hoencamp E, Zwaan T: Interpersoonlijke psychotherapie voor depressie. Een pilot-onderzoek. Tijdschr Psychiatr. 1996, 38: 398-402.
Markowitz JC, Kocsis JH, Bleiberg KL, Christos PJ, Sacks M: A comparative trial of psychotherapy and pharmacotherapy for "pure" dysthymic patients. J Affect Disord. 2005, 89: 167-175. 10.1016/j.jad.2005.10.001.
Browne G, Steiner M, Roberts J, Gafni A, Byrne C, Dunn E, Bell B, Mills M, Chalklin L, Wallik D, et al: Sertraline and/or interpersonal psychotherapy for patients with dysthymic disorder in primary care: 6-month comparison with longitudinal 2-year follow-up of effectiveness and costs. J Affect Disord. 2002, 68: 317-330. 10.1016/S0165-0327(01)00343-3.
Markowitz JC: Psychotherapy of dysthymia. Am J Psychiatry. 1994, 151 (8): 1114-1121.
Markowitz JC: Psychotherapy for dysthymic disorder. Psychiatr Clin North Am. 1996, 19 (1): 133-149. 10.1016/S0193-953X(05)70278-1.
Svanborg C, Wistedt AA, Svanborg P: Long-term outcome of patients with dysthymia and panic disorder: a naturalistic 9-year follow-up study. Nord J Psychiatry. 2008, 62 (1): 17-24. 10.1080/08039480801960123.
Schulberg HC, Madonia MJ, Block MR, Coulehan JL, Scott CP, Rodriguez E, Black A: Major depression in primary care practice. Clinical characteristics and treatment implications. Psychosomatics. 1995, 36: 129-137. 10.1016/S0033-3182(95)71682-6.
Bulmash E, Harkness KL, Stewart JG, Bagby RM: Personality, stressful life events, and treatment response in major depression. J Consult Clin Psychol. 2009, 77: 1067-1077.
Kushner SC, Quilty LC, McBride C, Bagby RM: A comparison of depressed patients in randomized versus nonrandomized trials of antidepressant medication and psychotherapy. Depress Anxiety. 2009, 26: 666-673. 10.1002/da.20566.
Frank E, Grochocinski VJ, Spanier CA, Buysse DJ, Cherry CR, Houck PR, Stapf DM, Kupfer DJ: Interpersonal psychotherapy and antidepressant medication: Evaluation of a sequential treatment strategy in women with recurrent major depression. J Clin Psychiatry. 2000, 61 (1): 51-57. 10.4088/JCP.v61n0112.
Cuijpers P, Van Straten A, Warmerdam L, Andersson G: Psychological treatment of depression: a meta-analytic database of randomized studies. BMC Psychiatry. 2008, 8: 36-10.1186/1471-244X-8-36.
Dunner DL: Acute and maintenance treatment of chronic depression. J Clin Psychiatry. 2001, 62: 10-16.
Blanco C, Lipsitz J, Caligor E: Treatment of chronic depression with a 12-week program of interpersonal psychotherapy. Am J Psychiatry. 2001, 158: 371-375. 10.1176/appi.ajp.158.3.371.
Miller IW, Keitner GI: Combined medication and psychotherapy in the treatment of chronic mood disorders. Psychiatr Clin North Am. 1996, 19 (1): 151-171. 10.1016/S0193-953X(05)70279-3.
Reinceke MA, Ewell Foster CJ, Rogers GM, Weill R: Medication or psychotherapy for severe depression. Am J Psychiatry. 2000, 157 (9): 1528-1529. 10.1176/appi.ajp.157.9.1528.
Frank E, Kupfer DJ, Perel JM, Cornes C, Jarret DB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ: Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990, 47: 1093-1099. 10.1001/archpsyc.1990.01810240013002.
Bressi C, Porcellana M, Marinaccio PM, Nocito EP, Magri L: Short-term psychodynamic psychotherapy versus treatment as usual for depressive and anxiety disorders: a randomized clinical trial of efficacy. J Nerv Ment Dis. 2010, 198 (9): 647-652. 10.1097/NMD.0b013e3181ef3ebb.
Croghan TW, Melfi CA, Dobrez DG, Kniesner TJ: Effect of mental health specialty care on antidepressant length of therapy. Med Care. 1999, 37 (4 Suppl Lilly): AS20-AS23.
Cuijpers P, Van Lier PA, Van Straten A, Donker M: Examining differential effects of psychological treatment of depressive disorder: an application of trajectory analyses. J Affect Disord. 2005, 89 (1–3): 137-146.
Godfrin KA, Van Heeringen C: The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: A randomized controlled study. Behav Res Ther. 2010, 48 (8): 738-746. 10.1016/j.brat.2010.04.006.
Kingston T, Dooley B, Bates A, Lawlor E, Malone K: Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol Psychother. 2007, 80 (Pt 2): 193-203.
Schene AH, Koeter MW, Kikkert MJ, Swinkels JA, McCrone P: Adjuvant occupational therapy for work-related major depression works: randomized trial including economic evaluation. Psychol Med. 2007, 37 (3): 351-362. 10.1017/S0033291706009366.
Schulberg HC, Block MR, Madonia MJ, Scott CP, Lave JR, Rodriguez E, Coulehan JL: The 'usual care' of major depression in primary care practice. Arch Fam Med. 1997, 6 (4): 334-339. 10.1001/archfami.6.4.334.
Van Roijen LH, Van Straten A, Al M, Rutten F, Donker M: Cost-utility of brief psychological treatment for depression and anxiety. Br J Psychiatry. 2006, 188: 323-329. 10.1192/bjp.188.4.323.
Blom MBJ, Jonker K, Dusseldorp E, Spinhoven P, Hoencamp E, Haffmans J, Van Dyck R: Combination treatment for acute depression is superior only when psychotherapy is added to medication. Psychother Psychosom. 2007, 76: 289-297. 10.1159/000104705.
Luty SE, Carter JD, McKenzie JM, Rae AM, Frampton CMA, Mulder RT, Joyce PR: Randomised controlled trial of interpersonal psychotherapy and cognitive-behavioural therapy for depression. Br J Psychiatry. 2009, 190: 496-502.
Marshall MB, Zuroff DC, McBride C, Bagby RM: Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008, 64: 231-244. 10.1002/jclp.20438.
Martin SD, Martin E, Rai S, Richardson MA, Royall R: Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride. Arch Gen Psychiatry. 2001, 58: 641-648. 10.1001/archpsyc.58.7.641.
Schramm E, Zobel I, Dykierek P, Kech S, Brakemeier E, Külz A, Berger M: Cognitive behavioral analysis system of psychotherapy versus interpersonal psychotherapy for early-onset chronic depression: a randomized pilot study. J Affect Disord. 2011, 129: 109-116. 10.1016/j.jad.2010.08.003.
Schulberg HC, Block MR, Madonia MJ, Scott CP, Rodriguez E, Imber SD, Perel JM, Lave JR, Houck PR, Coulehan JL: Treating major depression in primary care practice. Eight-month clinical outcomes. Archives of General Psychiatry. 1996, 53: 913-919.
Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, Glass DR, Pilkonis PA, Leber WR, Docherty JP, et al: National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989, 46: 971-982. 10.1001/archpsyc.1989.01810110013002.
O'Hara MW, Stuart S, Gorman LL, Wenzel A: Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000, 57: 1039-1045. 10.1001/archpsyc.57.11.1039.
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised. 1987, Washington DC: American Psychiatric Association
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders (4th edition). 1994, Washington DC: American Psychiatric Association
McCullough JP: Treatment for Chronic Depression. Cognitive Behavioral Analysis System of Psychotherapy. 2000, New York: Guilford Press
Beck AT, Steer RA, Brown GK: Beck Depression Inventory: Manual. 1987, San Antonio: Harcourt Brace
Padesky CA, Greenberger D: Clinician's guide to mind over mood. 1995, New York: Guilford Press
Stewart DE: Hepatic adverse reactions associated with nefazodone. Can J Psychiatry. 2002, 47: 375-377.
Zimmerman M, Posternak MA, Chelminski I: Is it time to replace the Hamilton Depression Rating Scale as the primary outcome measure in treatment studies of depression?. J Clin Psychopharmacol. 2005, 25: 105-110. 10.1097/01.jcp.0000155824.59585.46.
Bagby RM, Ryder AG, Schuller DR, Marshall MB: The Hamilton Depression Rating Scale: has the gold standard become a lead weight?. Am J Psychiatry. 2004, 161: 2163-2177. 10.1176/appi.ajp.161.12.2163.
Santen G, Danhof M, Pasqua OD: Sensitivity of the Montgomery Asberg Depression Rating Scale to response and its consequences for the assessment of efficacy. J Psychiatr Res. 2009, 43: 1049-1056. 10.1016/j.jpsychires.2009.02.001.
Schotte CKW, Maes M, Cluydts R, De Doncker D, Cosyns P: Construct validity of the Beck Depression Inventory in a depressive population. J Affect Disord. 1997, 46: 115-125. 10.1016/S0165-0327(97)00094-3.
Richter P, Werner J, Heerlein A, Kraus A, Sauer H: On the validity of the Beck Depression Inventory. Psychopathology. 1998, 31: 160-168. 10.1159/000066239.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-244X/13/22/prepub
Download references
Acknowledgements
This study was not funded by any grants. We thank Tim Ellermann and Henrietta Hazen for help during the development of an adequate search strategy. MH also thanks SE and TR for their support.
Author information
Authors and affiliations.
Caphri, School of Public Health and Primary Care; Faculty of Health, Medicine, and Life Sciences, Maastricht University, Maastricht, The Netherlands
Madelon L J M van Hees, Thomas Rotter & Silvia M A A Evers
College of Pharmacy and Nutrition, University of Saskatchewan, Saskoon, Canada
Thomas Rotter
Institute for Public Health and Nursing Research (IPP), University of Bremen, Bremen, Germany
Tim Ellermann
Caphri, School of Public Health and Primary Care; Department of Health Services Research, Maastricht University, Maastricht, The Netherlands
Silvia M A A Evers
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Madelon L J M van Hees .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
MH designed the study with the support of SE and TR. MH undertook the literature search with help from TE, identified potential and final selected articles, interpreted results, drafted and revised all versions of the manuscript, supported by SE and TR. In case of doubt during the screening and analyzing phase, TR was consulted. SE and TR supervised the development of the manuscript. All authors read and approved the final version.
Electronic supplementary material
Additional file 1: search strategy.(doc 27 kb), additional file 2: checklist.(doc 24 kb), additional file 3: list of excluded studies.(doc 73 kb), authors’ original submitted files for images.
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, authors’ original file for figure 3, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
van Hees, M.L.J.M., Rotter, T., Ellermann, T. et al. The effectiveness of individual interpersonal psychotherapy as a treatment for major depressive disorder in adult outpatients: a systematic review. BMC Psychiatry 13 , 22 (2013). https://doi.org/10.1186/1471-244X-13-22
Download citation
Received : 09 December 2011
Accepted : 07 January 2013
Published : 11 January 2013
DOI : https://doi.org/10.1186/1471-244X-13-22
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Interpersonal psychotherapy
- Major depressive disorder
- Systematic review
BMC Psychiatry
ISSN: 1471-244X
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta‐analytic evaluation of recent meta‐analyses
Falk leichsenring, christiane steinert, sven rabung, john pa ioannidis.
- Author information
- Article notes
- Copyright and License information
Issue date 2022 Feb.
Mental disorders represent a worldwide public health concern. Psychotherapies and pharmacotherapies are recommended as first line treatments. However, evidence has emerged that their efficacy may be overestimated, due to a variety of shortcomings in clinical trials (e.g., publication bias, weak control conditions such as waiting list). We performed an umbrella review of recent meta‐analyses of randomized controlled trials (RCTs) of psychotherapies and pharmacotherapies for the main mental disorders in adults. We selected meta‐analyses that formally assessed risk of bias or quality of studies, excluded weak comparators, and used effect sizes for target symptoms as primary outcome. We searched PubMed and PsycINFO and individual records of the Cochrane Library for meta‐analyses published between January 2014 and March 2021 comparing psychotherapies or pharmacotherapies with placebo or treatment‐as‐usual (TAU), or psychotherapies vs. pharmacotherapies head‐to‐head, or the combination of psychotherapy with pharmacotherapy to either monotherapy. One hundred and two meta‐analyses, encompassing 3,782 RCTs and 650,514 patients, were included, covering depressive disorders, anxiety disorders, post‐traumatic stress disorder, obsessive‐compulsive disorder, somatoform disorders, eating disorders, attention‐deficit/hyperactivity disorder, substance use disorders, insomnia, schizophrenia spectrum disorders, and bipolar disorder. Across disorders and treatments, the majority of effect sizes for target symptoms were small. A random effect meta‐analytic evaluation of the effect sizes reported by the largest meta‐analyses per disorder yielded a standardized mean difference (SMD) of 0.34 (95% CI: 0.26‐0.42) for psychotherapies and 0.36 (95% CI: 0.32‐0.41) for pharmacotherapies compared with placebo or TAU. The SMD for head‐to‐head comparisons of psychotherapies vs. pharmacotherapies was 0.11 (95% CI: –0.05 to 0.26). The SMD for the combined treatment compared with either monotherapy was 0.31 (95% CI: 0.19‐0.44). Risk of bias was often high. After more than half a century of research, thousands of RCTs and millions of invested funds, the effect sizes of psychotherapies and pharmacotherapies for mental disorders are limited, suggesting a ceiling effect for treatment research as presently conducted. A paradigm shift in research seems to be required to achieve further progress.
Keywords: Psychotherapies, pharmacotherapies, mental disorders, randomized controlled trials, meta‐analyses, effect sizes, meta‐analytic evaluation
Mental disorders represent a worldwide public health concern 1 , 2 . Psychotherapies and pharmacotherapies are recommended as first line treatments 3 , 4 . However, evidence has recently emerged suggesting that the efficacy of both types of treatment may have been overestimated, due to several shortcomings of clinical trials, such as publication bias, researcher allegiance, or use of weak comparison groups (in particular, waiting list) 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 . A realistic estimate of the efficacy of psychotherapies and pharmacotherapies is important to obtain.
Meta‐analyses and systematic reviews of randomized controlled trials (RCTs) are thought to provide the highest level of evidence 17 . However, not only individual RCTs but also meta‐analyses may be affected by the above‐mentioned biases 6 , 18 , 19 . To avoid overestimating treatment efficacy, meta‐analyses need to take risk of bias systematically into account 6 , 18 , 19 , 20 . Furthermore, estimates of efficacy depend upon the comparator against which treatments are tested. Waiting list conditions, for example, represent a relatively weak comparator, leading to larger effect sizes 6 , 8 , 21 , 22 . Comparisons with placebo or treatment‐as‐usual (TAU) provide better estimates of the true efficacy of a treatment 6 , 22 .
The most recent comprehensive review of meta‐analyses of both psychotherapies and pharmacotherapies in mental disorders, including 61 meta‐analyses, was published in 2014, reporting a medium effect size (standardized mean difference, SMD = 0.50) 8 . Some of the included meta‐analyses, however, used waiting list comparisons in the assessment of overall efficacy. In addition, the authors seem to have just averaged the extracted effect sizes, without performing a meta‐analytic evaluation including weighting effect sizes. Furthermore, a large number of studies and meta‐analyses have been published since 2014.
For all these reasons, we carried out an up‐to‐date umbrella review of recent meta‐analyses of psychotherapies and pharmacotherapies for the main mental disorders in adults which used placebo or TAU as comparison groups and formally assessed risk of bias or quality of studies. As the primary outcome, we used the effect size for target symptoms of the relevant disorder.
The study protocol of this umbrella review was registered in advance at PROSPERO (International Prospective Register of Systematic Reviews), registration number: CRD42020155452.
Inclusion criteria
Meta‐analyses of RCTs comparing psychotherapies or pharmacotherapies to placebo or TAU in adults with mental disorders published since 2014 were eligible. We also considered meta‐analyses comparing psychotherapies vs. pharmacotherapies head‐to‐head, or their combination to either monotherapy. Only meta‐analyses which formally assessed risk of bias or quality of studies were included. If multiple meta‐analyses fulfilling the inclusion criteria were available for one condition, all of them were included. Reporting of SMD or other measures of between‐group effect size was required.
All types of pharmacotherapy or psychotherapy were eligible for inclusion. Meta‐analyses examining specific subgroups (e.g., treatment resistant depression, primary care patients, the elderly), psychiatric or somatic comorbidities (e.g., depression in cancer patients), specific settings (e.g., group therapy only, or inpatient therapy) or augmentation strategies (e.g., psychostimulants added to antipsychotic drugs in schizophrenia), or focusing on secondary outcomes (e.g., quality of life in depression) were not included. These inclusion criteria are consistent with the above‐mentioned 2014 review 8 , except for excluding waiting list comparisons and requiring meta‐analyses to have assessed risk of bias or quality of studies. Both standard and network meta‐analyses were eligible.
Combining data of patients receiving TAU or placebo with those of patients on waiting list has been shown to inflate effect sizes 8 , 22 , 23 . On the other hand, mixing data of patients on TAU with those receiving specific therapies (e.g., cognitive‐behaviour therapy, CBT) can be expected to underestimate the effect size of the treatment in question. Therefore, meta‐analyses mixing data of TAU or placebo with waiting list or active treatments were excluded.
Search for studies
We searched PubMed and PsycINFO and individual records of the Cochrane Library for meta‐analyses of RCTs of psychotherapies and/or pharmacotherapies for mental disorders in adults published between January 2014 and March 2021.
Four reviewers independently searched for studies. Decision on inclusion was made by consensus including another rater. Search terms were meta‐analy* or metaanaly* combined with the thesaurus of the individual databases concerning each disorder. To provide comparable results, we used the syntax applied in the previous most comprehensive review 8 .
Data extraction
We focused on effect sizes and 95% confidence intervals (CIs) for the target symptoms of the relevant disorder (primary outcome). We extracted between‐group SMDs and related measures (Cohen's d, Hedges' g) as reported in the meta‐analyses. Odds ratios (ORs) and hazard ratios (HRs) were converted to SMDs 24 , 25 . Data on relative risk (RR) were extracted as reported. We used Cohen's convention of d=0.2, d=0.5 and d=0.8 as indicating small, medium and large effect sizes 26 , corresponding to success rate differences of 11%, 28% and 43%; numbers needed to treat of 9, 4 and 2; ORs of 1.43, 2.48 and 4.25; RRs of 1.22, 1.86 and 3.00; and HRs of 1.3, 1.9 and 2.8 24 , 25 , 27 , 28 , 29 . Intention‐to‐treat data were preferred whenever available.
If meta‐analyses took risk of bias into account by, for example, additionally reporting data separately for low risk of bias studies or correcting for publication bias, we listed all reported effect sizes but preferably focused on the corrected or high quality data for interpreting results.
Rates of remission and response were included as secondary outcomes when available. Dichotomous variables have some limitations 30 , but complementarily to SMDs they can provide useful information about efficacy.
One author extracted data (type of treatment and disorder, number of studies, number of participants, type of comparator, risk of bias, adverse events/side effects, and effect sizes). Data were cross‐checked independently by two raters each.
Quality assessment
The quality of the included meta‐analyses was independently assessed by two raters. For the purpose of this review, we used the items 1 to 9 of the Checklist for Systematic Reviews and Research Syntheses 31 , 32 , complemented by item 12 of AMSTAR 2 20 (“Was the impact of risk of bias in individual studies on results of the meta‐analysis taken into account?”) and an additional item addressing whether the meta‐analysis was registered. In case of disagreement between raters, consensus ratings were used.
Data synthesis
The results of the largest meta‐analyses for each condition, i.e. those including most RCTs, are presented and evaluated separately. Additionally, these independent meta‐analyses were included in second‐order meta‐analyses combining their summary effect sizes across all the different mental disorders 33 . This allowed to obtain a weighted effect of psychotherapy or pharmacotherapy across all mental disorders, and weighted effects for the benefits of combined therapy, and for the comparative efficacy of psychotherapy vs. pharmacotherapy. The analysis was performed by Comprehensive Meta‐Analysis (CMA, Version 3) using a random effects model based on SMDs and their CIs via the CMA analysis option “generic estimates”.
Heterogeneity was assessed using the I 2 statistic. If meta‐analyses did not report an overall effect size, but effect sizes for specific treatments and comparisons, the effect sizes of the relevant comparisons were aggregated by CMA and the resulting overall SMDs were entered into the second‐order meta‐analyses across disorders. Only effect estimates based on at least two primary RCTs were used.
Included meta‐analyses
The search retrieved 23,601 items, reduced to 19,500 after removing duplicates, which were screened by titles and abstracts. Full‐text evaluation was carried out for 319 papers. One hundred and two meta‐analyses fulfilled the inclusion criteria (see Figure 1 and supplementary information). These encompassed 69 meta‐analytic comparisons of pharmacotherapies with placebo or TAU, 26 comparisons of psychotherapies with placebo or TAU, 11 comparisons of psychotherapies vs. pharmacotherapies head‐to‐head, and 13 comparisons of combined psychotherapy and pharmacotherapy to either monotherapy 6 , 12 , 13 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 . The 102 meta‐analyses encompassed 3,782 RCTs (range: 2 to 522) and 650,514 patients (range: 65 to 116,477) (see supplementary information).
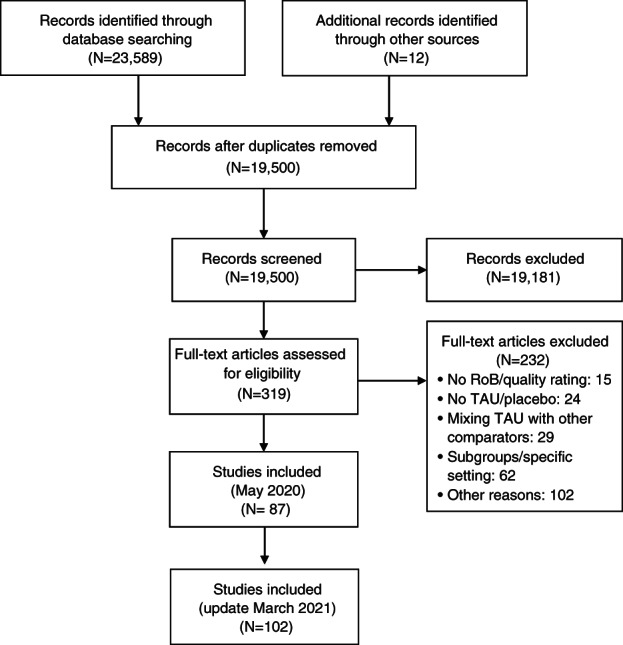
PRISMA flow chart. RoB – risk of bias, TAU – treatment as usual
Across all meta‐analyses, the mean number of positively rated items in the quality assessment was 8.71±1.43 (range: 4 to 11). The items 10 (item 12 of AMSTAR 2, addressing whether the meta‐analyses took the impact of bias on results into account) and 11 (study registration) were the least frequently fulfilled (48% and 47%, respectively). The quality of meta‐analyses was not significantly different between psychotherapies and pharmacotherapies (mean of positively rated items: 8.95±1.12 for psychotherapies, 8.68±1.54 for pharmacotherapies, t=0.74, p=0.46).
Psychotherapies and pharmacotherapies vs. TAU or placebo
In the largest meta‐analyses, the effect sizes of both psychotherapies and pharmacotherapies in comparison to TAU or placebo were small (SMD<0.50) for most disorders and treatments (see Figure 2 and supplementary information). Medium effect sizes were found only for pharmacotherapies of obsessive‐compulsive disorder (OCD) (SMD=0.56) 72 , bulimia nervosa (SMD=0.61) 80 , and somatoform disorders (SMD=0.50) 91 , and for psychotherapies of post‐traumatic stress disorder (PTSD) (SMD=0.54) 54 and borderline personality disorder (SMD=0.57) 93 . Large effect sizes were only reported for psychotherapy of OCD (SMD=1.03) 74 , with, however, a substantial proportion of patients taking concomitant pharmacotherapy 72 , 74 . Overall, risk of bias was often high (see Figure 2 and supplementary information).
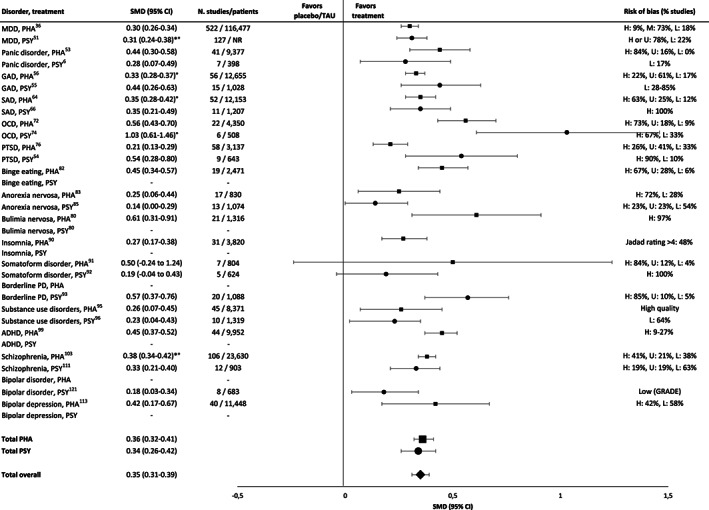
Effect sizes in the largest meta‐analyses of pharmacotherapies (squares) and psychotherapies (circles) in comparison to placebo or treatment‐as‐usual (TAU). PHA – pharmacotherapy; PSY – psychotherapy, SMD – standardized mean difference, * – adjusted for risk of bias, ° – adjusted for small‐study effects, MDD – major depressive disorder, GAD – generalized anxiety disorder, SAD – social anxiety disorder, OCD – obsessive‐compulsive disorder, PTSD – post‐traumatic stress disorder, PD – personality disorder, ADHD – attention‐deficit/hyperactivity disorder, H – high, M – medium, L – low, U – uncertain, NR – not reported. Where SMD is not provided, this means that no valid meta‐analysis reporting this value was available.
For psychotherapies and pharmacotherapies, second‐order random effects meta‐analyses in comparison to placebo or TAU yielded statistically significant but small SMDs of 0.34 (95% CI: 0.26‐0.42, I 2 =66.33%) and 0.36 (95% CI: 0.32‐0.41, I 2 =70.61%), respectively, across disorders (see Figure 2 ). For the aggregated data of psychotherapies and pharmacotherapies, the SMD was 0.35 (95 CI: 0.31‐0.39, I 2 =68.23%).
Depressive disorders
For psychotherapies of depressive disorders, the largest meta‐analysis reported a small SMD of 0.31, adjusted for biases, in comparison to TAU 51 (see Figure 2 ). Taking all included meta‐analyses into account, psychotherapy achieved effect sizes (SMDs) between 0.11 and 0.61 in comparison to placebo or TAU 6 , 12 , 37 , 50 , 51 , 52 , except for one outlying meta‐analysis reporting a large SMD post‐therapy (1.11), reduced to 0.27 at 3 to 12 month follow‐up and associated with a high risk of bias 52 . The majority of effect sizes were small (<0.50).
Only between 1% and 17% of studies of psychotherapy for depression were found to show a low risk of bias. When meta‐analyses took risk of bias into account, they consistently found a decrease in effect sizes (see supplementary information).
Across all forms of psychotherapy, remission from major depressive disorder (Hamilton Depression Rating Scale, HAM‐D <7) was achieved in 43% of patients, with no significant differences between the various psychotherapies 5 . Response (50% reduction of HAM‐D score) was achieved in 54% of patients 5 . TAU was superior to no treatment with regard to remission (33% vs. 23%), but inferior to psychotherapy (33% vs. 43%) 135 .
The largest meta‐analysis of pharmacotherapy for depressive disorders reported a SMD of 0.30 36 (see Figure 2 ). All effect sizes (SMD) achieved by pharmacotherapy in comparison to placebo were below 0.50, ranging from 0.19 to 0.41. The exception was ketamine, which achieved large short‐term effects (0.83, 0.88) 24 hours and 3‐4 days after treatment, dropping to 0.31 after 7 days 13 , 34 , 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 . Most effect sizes in terms of RRs were small as well (≤1.22).
The mean response rate for selective serotonin reuptake inhibitors (SSRIs) was 51% vs. 39% for placebo 35 , corresponding to a small effect size 27 .
Many trials of pharmacotherapy in depression showed a high risk of bias 13 , 35 , 36 , 42 (see supplementary information).
Anxiety disorders
In the largest meta‐analyses of anxiety disorders, psychotherapies achieved SMDs between 0.28 and 0.44 6,55,66 (see Figure 2 ). Overall, psychotherapies of anxiety disorders achieved SMDs compared to TAU or placebo between 0.01 and 0.72 6 , 54 , 55 , 59 , 65 , 66 , 71 , except for two outlying effect sizes in generalized anxiety disorder (1.44, 1.32), each based on three studies only 6 , 55 . Two effect sizes of psychotherapy (CBT) in social anxiety disorder were medium (0.72, 0.56) 66 , but most effect sizes were small (see supplementary information).
Overall, only 17% of psychotherapy studies in anxiety disorders were found to show a low risk of bias 6 .
In the largest meta‐analyses for anxiety disorders, pharmacotherapies achieved SMDs in comparison to placebo between 0.33 and 0.45 53,56,64 (see Figure 2 ). Overall, effect sizes for pharmacotherapy were between 0.01 and 0.56, with the majority of effect sizes being small 53 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 66 , 67 , 69 , 70 (see supplementary information). RR ranged between 1.20 and 4.03, with most values being small, one medium (monoamine oxidase inhibitors), and one large (benzodiazepines, RR=4.03) 69 .
For social anxiety disorder and generalized anxiety disorder, pharmacotherapy yielded response rates of 52% and 56%, respectively, versus 32% and 41% with placebo 59 , 69 .
Obsessive‐compulsive disorder
For psychotherapy (CBT) of OCD, the largest meta‐analysis reported a large SMD (1.03) 74 (see Figure 2 ). Considering all meta‐analyses, large SMDs in comparison to placebo were reported (0.91‐1.46) 72 , 74 . At follow‐up of on average of 15.1 months after the end of treatment, SMDs decreased from 0.57 to 0.06 for all comparators 74 . Follow‐up results were not available for a comparison against placebo. Most psychotherapy trials included patients taking stable doses of antidepressants 72 , 74 , possibly overestimating effect sizes in favour of psychotherapy 72 .
For pharmacotherapy of OCD, the largest meta‐analysis reported a medium effect size (SMD=0.56) 72 (see Figure 2 ). Considering all meta‐analyses, small to medium SMDs were reported (0.45‐0.66).
For most studies of psychotherapy and pharmacotherapy, the risk of bias was high (see Figure 2 and supplementary information).
Post‐traumatic stress disorder
For psychotherapy (CBT) of PTSD, the largest meta‐analysis reported a medium effect size compared to TAU (SMD=0.54) 54 (see Figure 2 ), which was stable at follow‐ups of up to 12 months after end of therapy 54 . For PTSD related to childhood maltreatment, a SMD of 0.50 in comparison to TAU/placebo was found, which was reduced to 0.21 after adjusting for small sample size 79 .
For pharmacotherapy of PTSD, the largest meta‐analysis reported a small SMD in comparison to placebo (0.21) 76 (see Figure 2 ). Considering all meta‐analyses, effect sizes achieved by pharmacotherapy in comparison to placebo were heterogeneous (SMDs: –0.10 to 0.97) 75 , 76 , 77 , 78 . Risk of bias was often high 77 , 78 . A large SMD was obtained with phenelzine (0.97), a medium one with mirtazapine (0.79), desipramine (0.52) and olanzapine (0.51), all based on only one RCT except for olanzapine 75 . For SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs), a medium SMD was reported (0.50) 77 . For all other drugs, SMDs were <0.50 (from –0.10 to 0.47).
Personality disorders
For psychotherapy of personality disorders, only a meta‐analysis of borderline personality disorder was available, which reported a medium SMD in comparison to TAU (0.57), with a high risk of bias in most studies (see Figure 2 ) 93 .
An update for developing a Cochrane report of pharmacotherapy in borderline personality disorder did not provide meta‐analytic results since the authors did not find robust evidence 136 .
Somatoform disorders
For psychotherapy of somatoform disorders, the largest meta‐analysis reported a small SMD (0.19, see Figure 2 ) in comparison to enhanced care, with high risk of bias due to lack of blinding 92 . For pharmacotherapy of somatoform disorders, the largest meta‐analysis reported a medium SMD (0.50, see Figure 2 ) in comparison with placebo 91 .
Considering all meta‐analyses, heterogeneous SMDs (from 0.13 to 0.91) were reported for pharmacotherapy, based on two or three RCTs, with a high risk of bias for most RCTs 91 .
Eating disorders
For psychotherapy of bulimia nervosa, no recent meta‐analysis fulfilled the inclusion criteria. For pharmacotherapy, the largest meta‐analysis reported a medium SMD in comparison with placebo (0.61, see Figure 2 ) 80 . Considering all meta‐analyses, considerable heterogeneity among classes of drugs were found (SMDs: 0.10‐1.00) 80 .
For psychotherapy of binge eating disorder, no meta‐analysis fulfilled the inclusion criteria. For pharmacotherapy, the largest meta‐analysis reported a small to medium SMD in comparison with placebo (0.45, see Figure 2 ) 82 . Considering all meta‐analyses, a small to medium effect size compared to placebo was found for pharmacotherapy (SMD=0.45, RR: 1.67, 2.61) 81 , 82 . One of these meta‐analyses reported a high 82 , the other a medium to low risk of bias 81 .
For psychotherapy of anorexia nervosa, the largest meta‐analysis reported a small SMD in comparison with TAU (0.14, see Figure 2 ) 85 . Overall, the effect sizes in comparison to TAU or placebo were small (SMD=0.10‐0.31, RR: 0.97, 1.28) 84 , 85 , 86 . For pharmacotherapy, the largest meta‐analysis reported a small effect size (SMD=0.25) 83 .
Substance use disorders
For both psychotherapy and pharmacotherapy of substance use disorders, the largest meta‐analysis reported small SMDs in comparison with TAU or placebo (0.23 and 0.26, respectively, see Figure 2 ) 95 , 96 . For psychotherapy, the effect size decreased at follow‐ups ≥8 months after end of treatment (SMD=0.05) 96 . Considering all meta‐analyses, small effect sizes were found for pharmacotherapy (SMDs: 0.07 to 0.35, RR: 0.32‐1.39) 94 , 95 .
For psychotherapy, no recent meta‐analysis fulfilled the inclusion criteria. The quality of studies was found to be low 137 . For pharmacotherapy, the largest meta‐analysis reported a small SMD in comparison with placebo (0.27, see Figure 2 ) 90 . Overall, for pharmacotherapy of insomnia, small to medium SMDs were reported (0.07 to 0.58) 88 , 89 , 90 . One meta‐analysis provided effect sizes only for one of eight outcome measures, with large SMDs (0.88‐1.38), suggesting selective reporting 87 .
Attention‐deficit/hyperactivity disorder (ADHD)
For psychotherapy of ADHD in adults, no meta‐analysis could be included 134 . For pharmacotherapy, the largest meta‐analysis reported a small to medium SMD in comparison with placebo (0.45, see Figure 2 ) 99 . Considering all meta‐analyses, the effects of pharmacotherapy were heterogeneous (from 0.16 to 0.97) 98 , 99 , 100 , 101 , 102 . Large SMDs were found for amphetamines 98 , 100 , 102 , small to medium SMDs for methylphenidate 100 , 101 . Risk of bias was often high or unclear, and level of evidence was low to very low 98 , 100 .
Schizophrenia spectrum disorders
Results of psychotherapy in schizophrenia spectrum disorders were evaluated in the context of pharmacotherapy (i.e., patients usually received concomitant medication). The largest meta‐analysis reported a small SMD in comparison with TAU (0.33, see Figure 2 ) 111 . Considering all meta‐analyses, small effect sizes compared to nonspecific controls were found for overall symptoms, positive and negative symptoms (SMDs: 0.32, 0.24 and 0.08, respectively) 138 . In comparison to TAU, psychotherapy achieved small to medium SMDs for negative symptoms (0.15‐0.58) 110 , 112 .
For psychotherapy, a response rate of 13% for overall symptoms and 25% for positive symptoms was found 139 (a reduction of symptoms of at least 50% was required). The response rate decreased considerably if researcher allegiance (authors evaluated the therapy that they developed) was taken into account (from 13% to 4.9%) 139 .
For acute pharmacological treatment of schizophrenia, the largest meta‐analysis reported an overall SMD of 0.45 for target symptoms, reduced to 0.38 after adjusting for publication bias (Figure 2 ) 103 . These results are consistent with meta‐analyses on specific drugs, such as quetiapine (SMD=0.33), cariprazine (SMD: 0.32‐0.37), lurasidone (SMD: 0.34‐0.47), and aripiprazole and brexpiprazole (RR=1.1) 104 , 107 , 108 , 109 . A large effect size was reported for clozapine (SMD=0.89) 103 . Large and medium SMDs were achieved by long‐acting injectable antipsychotics in the maintenance treatment of non‐affective psychoses (RR: 1.75‐3.70) 107 .
For the acute treatment of schizophrenia with pharmacotherapy, differences in response rates in comparison with placebo were small (23% vs. 14%) 14 .
Bipolar disorder
For psychotherapy of bipolar disorder, the largest meta‐analysis reported a small effect size in comparison to TAU (SMD=0.18, see Figure 2 ) 121 , with small effect sizes for both depression and mania symptoms (SMDs: 0.23 and 0.05, respectively) and for relapse prevention post‐therapy (RR: 1.52) 121 . At follow‐up 26 to 78 weeks post‐therapy, SMDs were 0.21 and 0.38; RR for relapse was 1.35 121 . Psychotherapy was given in the context of concomitant pharmacotherapy.
For the acute treatment of mania, the results for pharmacotherapy are heterogeneous. One meta‐analysis reported medium SMDs for cariprazine (0.51‐0.52) 119 , and another reported a small effect size for aripiprazole (SMD=0.16) 115 . These two meta‐analyses included only three RCTs. A third meta‐analysis reported a very large SMD (1.51) for tamoxifen 120 , based on two RCTs with small samples (16 and 66 cases, respectively), making the results questionable. This study represents a clear outlier.
For the acute treatment of bipolar depression, the largest meta‐analysis of pharmacotherapy reported heterogeneous results, with effect sizes (SMDs) between 1.41 and –1.84 113 . Large effect sizes were achieved by fluoxetine (1.41), divalproex (1.25), lurasidone (1.15), moclobemide (1.09), cariprazine (0.85) and imipramine (0.86) 113 , all of them based, however, on only 0‐3 direct comparisons. Some drugs achieved medium effect sizes (olanzapine, phenelzine, tranylcypromine). The effect sizes of all other drugs were small 113 . Quetiapine achieved an almost medium effect size (SMD=0.48) based on 11 direct comparisons 113 .
For the prevention of manic/hypomanic/mixed episodes, effect sizes of lithium were almost medium (RR=1.85) 114 . Medium effect sizes were reported for olanzapine (RR=2.88) and risperidone (RR=2.88); large effect sizes for aripiprazole (once monthly) and asenapine (RR: 3.31, 4.81) 114 . The results for asenapine are based on only one RCT 116 . For all other drugs, effect sizes were small 114 .
For the prevention of any mood episode relapse, a large effect size was found for asenapine (RR=3.82), with the caveat mentioned above 114 . Medium effect sizes were reported for quetiapine and olanzapine 114 , 116 , 118 . Small effect sizes for the prevention of any mood episode were achieved by lithium (RR: 1.60, 1.61) and several other drugs 114 , 118 . Earlier meta‐analyses reported heterogeneous results for the prevention of any relapse by lithium (SMD: 1.12, 0.47) 140 .
For the prevention of depressive episodes, quetiapine and asenapine achieved medium effect sizes (RR: 2.08, 2.60). For all other drugs, including lithium (RR=1.26), effect sizes were small 114 . Small effect sizes were found for antidepressants (RR=1.56) 117 .
Psychotherapies vs. pharmacotherapies
Head‐to‐head comparisons of psychotherapies vs. pharmacotherapies yielded small effect sizes for all disorders examined, i.e., depressive disorders, anxiety disorders, PTSD and OCD (SMDs: 0.00‐0.24, see Figure 3 ) 37 , 66 , 74 , 126 . A second‐order random effects meta‐analysis across the effect sizes of the largest meta‐analyses (Figure 3 ) yielded a non‐significant SMD of 0.11 (95% CI: –0.05 to 0.26, I 2 =61.99).
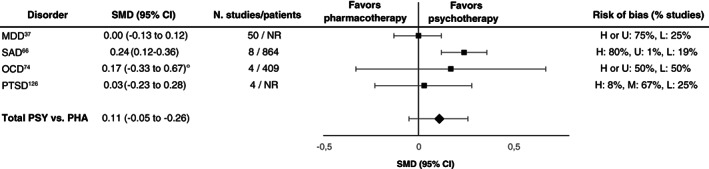
Effect sizes in the largest meta‐analyses for head‐to‐head comparisons of psychotherapies (PSY) vs. pharmacotherapies (PHA). SMD – standardized mean difference, ° – adjusted for small‐study effects, MDD – major depressive disorder, SAD – social anxiety disorder, OCD – obsessive‐compulsive disorder, PTSD – post‐traumatic stress disorder, H – high, M – medium, L – low, U – uncertain, NR – not reported
Considering all included meta‐analyses, no substantial differences in short‐term efficacy between psychotherapies and pharmacotherapies in depressive disorders, anxiety disorders and PTSD were found 12 , 37 , 66 , 122 , 123 , 124 , 125 , 126 , with only a few exceptions. In OCD, psychotherapy achieved medium to large SMDs (0.61‐0.95) in comparison to SSRIs 72 , but most psychotherapy trials included patients taking stable doses of antidepressants, affecting results in favour of psychotherapy. Most studies of psychotherapy and pharmacotherapy in OCD had a high risk of bias 72 . With regard to long‐term efficacy, psychotherapy achieved a large SMD compared to pharmacotherapy in PTSD (0.83) 126 . For other disorders, no head‐to‐head comparisons fulfilled the inclusion criteria.
Combining psychotherapy and pharmacotherapy
In the largest meta‐analyses (Figure 4 ), effect sizes (SMDs) in favour of the combined treatment were small for most disorders, that is depressive disorders (0.37, 0.15) 37 , social anxiety disorder (combined vs. pharmacotherapy: 0.40) 66 , OCD (combined vs. psychotherapy: 0.25) and PTSD (0.09, 0.12) 126 . Effect sizes (SMDs) were medium in favour of the combined treatment vs. psychotherapy in social anxiety disorder (0.52) 66 and for the combined treatment vs. pharmacotherapy (SSRIs) in OCD (0.73), based on a network meta‐analysis including only one direct comparison with very small samples 72 . A large effect size was found only for the combined treatment vs. pharmacotherapy in ADHD (0.80) 134 , based on only two RCTs showing a high risk of bias in at least one domain.
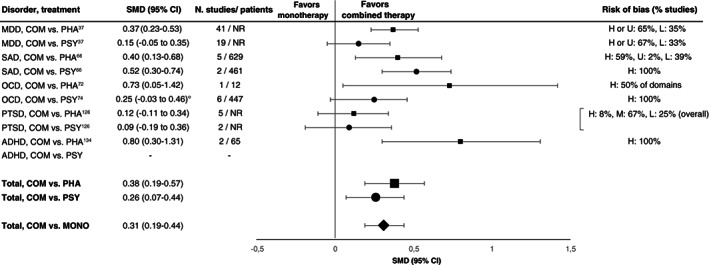
Effect sizes in the largest meta‐analyses for combined therapy vs. pharmacological (squares) or psychological (circles) monotherapy. SMD – standardized mean difference, ° – adjusted for small‐study effects, COM – combined therapy, PHA – pharmacotherapy, PSY – psychotherapy, MONO – monotherapy, MDD – major depressive disorder, SAD – social anxiety disorder, OCD – obsessive‐compulsive disorder, PTSD – post‐traumatic stress disorder, ADHD – attention‐deficit/hyperactivity disorder, H – high, M – medium, L – low, U – uncertain, NR – not reported
A second‐order random effects meta‐analysis across the effect sizes of the largest meta‐analyses yielded a statistically significant but small SMD of 0.31 (95% CI: 0.19‐0.44, I 2 =53.02) in favour of the combined treatment (Figure 4 ).
Considering all included meta‐analyses, most effect sizes (SMDs) achieved by the combined treatment compared to either monotherapy in depressive disorders, anxiety disorders, PTSD, OCD and ADHD were small (0.09‐0.48) when risk of bias was taken into account 12 , 37 , 66 , 72 , 74 , 126 , 128 , 129 , 131 , 132 , 134 . Exceptions were the superiority of the combined treatment in long‐term outcome of PTSD over pharmacotherapy (SMD=0.96, based on only two direct comparisons) 126 , and the superiority of the combined treatment over psychodynamic therapy in social anxiety disorder (SMD=0.68), based on a network meta‐analysis including zero direct comparisons for the condition 66 , making a study of inconsistencies impossible 141 .
In several of these meta‐analyses, risk of bias was high in several domains, or results were based on only a few or small studies 66 , 72 , 126 , 134 .
In this field‐wide assessment of psychotherapies and pharmacotherapies for mental disorders in adults, we included evidence from 102 meta‐analyses with 3,782 RCTs and 650,514 patients. We found small benefits overall for both types of interventions, with an average SMD of 0.35 and moderate heterogeneity across conditions 142 . This finding challenges the result of the previous most comprehensive review, which reported an overall medium effect size (SMD=0.50) across psychotherapies and pharmacotherapies, based on 61 meta‐analyses with 852 RCTs and 137,126 patients 8 . This latter estimate seems to be due to including waiting list comparators and averaging effect sizes without performing a random effects meta‐analytic evaluation.
According to the results of this umbrella review and second‐order meta‐analyses, there is an additional gain of psychotherapy and pharmacotherapy in the treatment of mental disorders in adults, but this is small in terms of effect sizes 26 . Conditions for which very extensive evidence was available (e.g., depression) almost always had such modest effect sizes when only studies with low risk of bias were considered, or efforts were made to correct for bias. Medium or large effect sizes were found only for few conditions, and most of the effects sizes ≥0.50 were associated with a high risk of bias and/or limited evidence. Nevertheless, the argument still holds that, although there are some medications for general medical conditions with clearly higher effect sizes, psychotropic agents or psychotherapies are not generally less efficacious than those medications 140 .
Some limitations and features of this umbrella review should be discussed as they affect the interpretation of overall evidence. First, several meta‐analytic comparisons included only a few studies, affecting statistical power and external validity of results.
Second, the results of network meta‐analyses need some extra caution 141 , 143 . It has been argued that these meta‐analyses can only provide observational evidence, since the comparisons between treatments are both direct and indirect, and the latter are not randomized 144 . As a related issue, transitivity (similar distribution of effect modifiers) can be controlled statistically only for known modifiers, in contrast to controlling all modifiers by randomization. Some of the network meta‐analyses included in this review encompassed only a few or even no direct comparisons of specific treatments 66 , 72 , 75 . Statistical power may be low if only a few studies with small samples and large heterogeneity are included 141 , 145 . Thus, some inconsistencies between direct and indirect comparisons may not have been detected 145 , possibly affecting effect size estimates.
Third, we followed Cohen's convention of small, medium and large effect sizes 26 . However, the clinical relevance of these estimates is not clear. The clinical benefit of an intervention needs to be determined by comparison with a benchmark such as the minimal clinically important difference (MCID) 146 . For the HAM‐D, for example, a minimal clinically relevant improvement has been claimed by some authors to correspond to a 7‐point difference 147 or to an SMD of 0.88 148 . If this is correct, in psychotherapies or pharmacotherapies for depression, effect sizes of 0.30, 0.40 or even 0.50 correspond to a difference on the HAM‐D of 2 or 4 points (i.e. <7) which cannot be detected by clinicians and can therefore hardly be regarded as clinically significant. In schizophrenia, a SMD of 0.73 is required for a minimal clinical improvement of 15 points on the Positive and Negative Syndrome Scale (PANSS) 149 , implying that SMDs below 0.73 are not detectable by clinicians and may not be clinically significant.
For a better judgment, the CIs of the effect size estimates may be compared with the proposed MCID values 150 . It has been argued that, if the upper limit of the 95% CIs is smaller than the MCID, effect sizes can be regarded as “definitely clinically not important” 150 . For the vast majority of meta‐analyses on depression and schizophrenia, this would be the case if SMDs of 0.88 and 0.73 are used as MCID. However, even if the summary effect sizes are substantially smaller than the MCID, there is heterogeneity in treatment responses across patients. Therefore, a minority of patients may still achieve large benefits from treatment.
Fourth, identical effect sizes may have different clinical importance in different patient populations (e.g., according to disorders, gender or age) and for different outcomes (e.g., mortality) 151 . For outcomes including vital events (e.g., rates of suicide) small differences in success rates may be clinically important, whereas for continuous measures of (often transient) depression, anxiety or other symptoms, small differences of a few scale points may not 152 . Of the meta‐analyses on the treatment of depression, for example, only a few examined hard outcomes such as suicidal behaviour 41 , 42 , 44 . In the meta‐analyses on schizophrenia and bipolar disorder, data on suicidal behaviour were not reported, except for one meta‐analysis 118 . Future studies and meta‐analyses should include such important hard outcomes.
Fifth, TAU as a comparator was found to be superior to no treatment in depression (with regard to remission, 33% vs. 23%) 135 . However, TAU is a heterogeneous condition, and effect sizes achieved depend on the type of treatment actually delivered. Larger effect sizes may be achieved in comparison to weaker forms of TAU 23 , 153 . This applies to psychological placebo as well 154 .
Sixth, the results of RCTs may not necessarily represent real‐world effectiveness 155 . In clinical practice, patients often suffer from multiple mental disorders, and treatments are usually tailored to the individual patients' needs. This applies, for example, to treatment duration. Most of the treatments included in this review were short‐term 6 . Data on longer‐term treatments are widely missing from RCT research.
In summary, a systematic re‐assessment of recent evidence across multiple meta‐analyses on key mental disorders provided an overarching picture of limited additional gain for both psychotherapies and pharmacotherapies over placebo or TAU. A ceiling seems to have been reached with response rates ≤50% and most SMDs not exceeding 0.30‐0.40. Thus, after more than half a century of research, thousands of RCTs and millions of invested funds, the “trillion‐dollar brain drain” 2 associated with mental disorders is presently not sufficiently addressed by the available treatments. This should not be seen as a nihilistic or dismissive conclusion, since undoubtedly some patients do benefit from the available treatments. However, realistically facing the situation is a prerequisite for improvement. Pretending that everything is fine will not move the field forward 156 , nor will conforming and producing more similar findings 157 .
A paradigm shift in research seems to be required to achieve further progress. Suggestions for such a shift have recently been made 11 , for example, for improving methodological quality and replicability (e.g., open science 158 , 159 ), improving available treatments – e.g., by personalized management 160 , 161 , 162 , defining specific targets and outcomes 163 , considering response to previous treatments (staging) 164 , 165 , switching or augmentation strategies 166 – or developing new treatments (e.g., exploration of out‐of‐the box ideas and accidental discoveries 167 ). A focus on prevention (e.g., in educational or occupational settings) 168 , 169 may improve the situation as well.
Improving treatment strategies for mental disorders can be regarded as a central health challenge of the 21st century.
ACKNOWLEDGEMENTS
The authors are grateful to M. Huhn for providing effect sizes for the pharmacological treatment of schizophrenia. They thank J. Matzat, an official patient representative of the Federal Joint Committee in Germany, for approving the paper. They are also indebted to M. Wolf for his support in producing the figures, to S. Uysal for his help in the evaluation of the quality of the included meta‐analyses and the review of the extracted data, and to B. Leowald and L. Feix for support in searching for meta‐analyses. The paper is dedicated to the late H. Kächele, a pioneer of psychotherapy research. Supplementary information on the study is available at https://osf.io/yvg2c/ .
- 1. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry 2016;3:171‐8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Smith K. Trillion‐dollar brain drain – enormous costs of mental health problems in Europe not matched by research investment. Nature 2011;478:15. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Nathan PE, Gorman JM. A guide to treatments that work, 4th ed. New York: Oxford University Press, 2015. [ Google Scholar ]
- 4. National Institute for Health and Care Excellence . Mental health and behavioural conditions. https://www.nice.org.uk .
- 5. Cuijpers P, Karyotaki E, Weitz E et al. The effects of psychotherapies for major depression in adults on remission, recovery and improvement: a meta‐analysis. J Affect Disord 2014;159:118‐26. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Cuijpers P, Cristea IA, Karyotaki E et al. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta‐analytic update of the evidence. World Psychiatry 2016;15:245‐8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Ioannidis JP. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philos Ethics Humanit Med 2008;3:14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Huhn M, Tardy M, Spineli LM et al. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta‐analyses. JAMA Psychiatry 2014;71:706‐15. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Leichsenring F, Abbass A, Hilsenroth MJ et al. Biases in research: risk factors for non‐replicability in psychotherapy and pharmacotherapy research. Psychol Med 2017;47:1000‐11. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Ioannidis JP. Why most published research findings are false. PLoS Med 2005;2:e124. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Leichsenring F, Steinert C, Ioannidis JPA. Toward a paradigm shift in treatment and research of mental disorders. Psychol Med 2019;49:2111‐7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Driessen E, Hollon SD, Bockting CL et al. Does publication bias inflate the apparent efficacy of psychological treatment for major depressive disorder? A systematic review and meta‐analysis of US National Institutes of Health‐funded trials. PLoS One 2015;10:e0137864. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Munkholm K, Paludan‐Muller AS, Boesen K. Considering the methodological limitations in the evidence base of antidepressants for depression: a reanalysis of a network meta‐analysis. BMJ Open 2019;9:e024886. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Leucht S, Leucht C, Huhn M et al. Sixty years of placebo‐controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta‐analysis, and meta‐regression of efficacy predictors. Am J Psychiatry 2017;174:927‐42. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Turner EH, Matthews AM, Linardatos E et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 2008;358:252‐60. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Dragioti E, Karathanos V, Gerdle B et al. Does psychotherapy work? An umbrella review of meta‐analyses of randomized controlled trials. Acta Psychiatr Scand 2017;136:236‐46. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Oxford Centre for Evidence‐Based Medicine . Levels of evidence. https://www.cebm.ox.ac.uk .
- 18. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. New York: Wiley, 2009. [ Google Scholar ]
- 19. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Shea BJ, Reeves BC, Wells G et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Furukawa TA, Noma H, Caldwell DM et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta‐analysis. Acta Psychiatr Scand 2014;130:181‐92. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Michopoulos I, Furukawa TA, Noma H et al. Different control conditions can produce different effect estimates in psychotherapy trials for depression. J Clin Epidemiol 2020;132:59‐70. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Watts SE, Turnell A, Kladnitski N et al. Treatment‐as‐usual (TAU) is anything but usual: a meta‐analysis of CBT versus TAU for anxiety and depression. J Affect Disord 2015;175:152‐67. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Azuero A. A note on the magnitude of hazard ratios. Cancer 2016;122:1298‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Stat Med 2000;19:3127‐31. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum, 1988. [ Google Scholar ]
- 27. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry 2006;59:990‐6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Olivier J, May WL, Bell ML. Relative effect sizes for measures of risk. Commun Stat Theory Methods 2017;46:6774‐81. [ Google Scholar ]
- 29. Kraemer HC, Neri E, Spiegel D. Wrangling with p‐values versus effect sizes to improve medical decision‐making: a tutorial. Int J Eat Disord 2020;53:302‐8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332:1080. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Aromataris E, Fernandez R, Godfrey CM et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132‐40. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Joanna Briggs Institute . Checklist for Systematic Reviews and Research Syntheses. Adelaide: Joanna Briggs Institute, 2017. [ Google Scholar ]
- 33. Borenstein M, Hedges LV, Higgins JPT et al. Introduction to meta‐analysis. Chichester: Wiley, 2011. [ Google Scholar ]
- 34. Bai S, Guo W, Feng Y et al. Efficacy and safety of anti‐inflammatory agents for the treatment of major depressive disorder: a systematic review and meta‐analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2020;91:21‐32. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Barth M, Kriston L, Klostermann S et al. Efficacy of selective serotonin reuptake inhibitors and adverse events: meta‐regression and mediation analysis of placebo‐controlled trials. Br J Psychiatry 2016;208:114‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Cipriani A, Furukawa TA, Salanti G et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet 2018;391:1357‐66. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Cuijpers P, Noma H, Karyotaki E et al. A network meta‐analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry 2020;19:92‐107. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Guo X, McCutcheon RA, Pillinger T et al. The magnitude and heterogeneity of antidepressant response in depression: a meta‐analysis of over 45,000 patients. J Affect Disord 2020;276:991‐1000. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Humes EC, Brunoni AR. Comment on Fu, J . and Chen, Y. : The efficacy and safety of 5 mg/d vortioxetine compared to placebo for major depressive disorder: a meta‐analysis. Psychopharmacology 2017;234:903‐4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Fu J, Chen Y. The efficacy and safety of 5 mg/d vortioxetine compared to placebo for major depressive disorder: a meta‐analysis. Psychopharmacology 2015;232:7‐16. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Henssler J, Kurschus M, Franklin J et al. Long‐term acute‐phase treatment with antidepressants, 8 weeks and beyond: a systematic review and meta‐analysis of randomized, placebo‐controlled trials. J Clin Psychiatry 2018;79:15r10545. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Jakobsen JC, Katakam KK, Schou A et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta‐analysis and trial sequential analysis. BMC Psychiatry 2017;17:58. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Kishi T, Meltzer HY, Matsuda Y et al. Azapirone 5‐HT1A receptor partial agonist treatment for major depressive disorder: systematic review and meta‐analysis. Psychol Med 2014;44:2255‐69. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Koesters M, Ostuzzi G, Guaiana G et al. Vortioxetine for depression in adults. Cochrane Database Syst Rev 2017;7:CD011520. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Kryst J, Kawalec P, Mitoraj AM et al. Efficacy of single and repeated administration of ketamine in unipolar and bipolar depression: a meta‐analysis of randomized clinical trials. Pharmacol Rep 2020;72:543‐62. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Laoutidis ZG, Kioulos KT. Desvenlafaxine for the acute treatment of depression: a systematic review and meta‐analysis. Pharmacopsychiatry 2015;48:187‐99. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Meeker AS, Herink MC, Haxby DG et al. The safety and efficacy of vortioxetine for acute treatment of major depressive disorder: a systematic review and meta‐analysis. Syst Rev 2015;4:21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Pae CU, Wang SM, Han C et al. Vortioxetine: a meta‐analysis of 12 short‐term, randomized, placebo‐controlled clinical trials for the treatment of major depressive disorder. J Psychiatry Neurosci 2015;40:174‐86. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Taylor D, Sparshatt A, Varma S et al. Antidepressant efficacy of agomelatine: meta‐analysis of published and unpublished studies. BMJ 2014;348:g1888. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Cuijpers P, Turner EH, Mohr DC et al. Comparison of psychotherapies for adult depression to pill placebo control groups: a meta‐analysis. Psychol Med 2014;44:685‐95. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Cuijpers P, Karyotaki E, Reijnders M et al. Was Eysenck right after all? A reassessment of the effects of psychotherapy for adult depression. Epidemiol Psychiatr Sci 2019;28:21‐30. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Lopez‐Lopez JA, Davies SR, Caldwell DM et al. The process and delivery of CBT for depression in adults: a systematic review and network meta‐analysis. Psychol Med 2019;49:1937‐47. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Bighelli I, Castellazzi M, Cipriani A et al. Antidepressants versus placebo for panic disorder in adults. Cochrane Database Syst Rev 2018;4:CD010676. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. van Dis EAM, van Veen SC, Hagenaars MA et al. Long‐term outcomes of cognitive behavioral therapy for anxiety‐related disorders: a systematic review and meta‐analysis. JAMA Psychiatry 2020;77:265‐73. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Carl E, Witcraft SM, Kauffman BY et al. Psychological and pharmacological treatments for generalized anxiety disorder (GAD): a meta‐analysis of randomized controlled trials. Cogn Behav Ther 2020;49:1‐21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Gomez AF, Barthel AL, Hofmann SG. Comparing the efficacy of benzodiazepines and serotonergic anti‐depressants for adults with generalized anxiety disorder: a meta‐analytic review. Expert Opin Pharmacother 2018;19:883‐94. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. He H, Xiang Y, Gao F et al. Comparative efficacy and acceptability of first‐line drugs for the acute treatment of generalized anxiety disorder in adults: a network meta‐analysis. J Psychiatr Res 2019;118:21‐30. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Kong W, Deng H, Wan J et al. Comparative remission rates and tolerability of drugs for generalised anxiety disorder: a systematic review and network meta‐analysis of double‐blind randomized controlled trials. Front Pharmacol 2020;11:580858. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Li X, Zhu L, Su Y et al. Short‐term efficacy and tolerability of venlafaxine extended release in adults with generalized anxiety disorder without depression: a meta‐analysis. PLoS One 2017;12:e0185865. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Maneeton N, Maneeton B, Woottiluk P et al. Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta‐analysis of randomized controlled trials. Drug Des Devel Ther 2016;10:259‐76. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Qin B, Huang G, Yang Q et al. Vortioxetine treatment for generalised anxiety disorder: a meta‐analysis of anxiety, quality of life and safety outcomes. BMJ Open 2019;9:e033161. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Wang SM, Woo YS, Kim NY et al. Agomelatine for the treatment of generalized anxiety disorder: a meta‐analysis. Clin Psychopharmacol Neurosci 2020;18:423‐33. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Zhang Y, Huang G, Yang S et al. Duloxetine in treating generalized anxiety disorder in adults: a meta‐analysis of published randomized, double‐blind, placebo‐controlled trials. Asia Pac Psychiatry 2016;8:215‐25. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Curtiss J, Andrews L, Davis M et al. A meta‐analysis of pharmacotherapy for social anxiety disorder: an examination of efficacy, moderators, and mediators. Expert Opin Pharmacother 2017;18:243‐51. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Heeren A, Mogoase C, Philippot P et al. Attention bias modification for social anxiety: a systematic review and meta‐analysis. Clin Psychol Rev 2015;40:76‐90. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Mayo‐Wilson E, Dias S, Mavranezouli I et al. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2014;1:368‐76. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Liu X, Li X, Zhang C et al. Efficacy and tolerability of fluvoxamine in adults with social anxiety disorder: a meta‐analysis. Medicine 2018;97:e11547. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Liu H, Li X, Han B et al. Effects of cognitive bias modification on social anxiety: a meta‐analysis. PLoS One 2017;12:e0175107. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Williams T, Hattingh CJ, Kariuki CM et al. Pharmacotherapy for social anxiety disorder (SAnD). Cochrane Database Syst Rev 2017;10:CD001206. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Sugarman MA, Loree AM, Baltes BB et al. The efficacy of paroxetine and placebo in treating anxiety and depression: a meta‐analysis of change on the Hamilton Rating Scales. PLoS One 2014;9:e106337. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Carpenter JK, Andrews LA, Witcraft SM et al. Cognitive behavioral therapy for anxiety and related disorders: a meta‐analysis of randomized placebo‐controlled trials. Depress Anxiety 2018;35:502‐14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Skapinakis P, Caldwell D, Hollingworth W et al. A systematic review of the clinical effectiveness and cost‐effectiveness of pharmacological and psychological interventions for the management of obsessive‐compulsive disorder in children/adolescents and adults. Health Technol Assess 2016;20:1‐392. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Skapinakis P, Caldwell DM, Hollingworth W et al. Pharmacological and psychotherapeutic interventions for management of obsessive‐compulsive disorder in adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2016;3:730‐9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Öst LG, Havnen A, Hansen B et al. Cognitive behavioral treatments of obsessive‐compulsive disorder. A systematic review and meta‐analysis of studies published 1993‐2014. Clin Psychol Rev 2015;40:156‐69. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Cipriani A, Williams T, Nikolakopoulou A et al. Comparative efficacy and acceptability of pharmacological treatments for post‐traumatic stress disorder in adults: a network meta‐analysis. Psychol Med 2018;48:1975‐84. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. de Moraes Costa G, Zanatta FB, Ziegelmann PK et al. Pharmacological treatments for adults with post‐traumatic stress disorder: a network meta‐analysis of comparative efficacy and acceptability. J Psychiatr Res 2020;130:412‐20. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Lee DJ, Schnitzlein CW, Wolf JP et al. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta‐analyses to determine first‐line treatments. Depress Anxiety 2016;33:792‐806. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Hoskins M, Pearce J, Bethell A et al. Pharmacotherapy for post‐traumatic stress disorder: systematic review and meta‐analysis. Br J Psychiatry 2015;206:93‐100. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Ehring T, Welboren R, Morina N et al. Meta‐analysis of psychological treatments for posttraumatic stress disorder in adult survivors of childhood abuse. Clin Psychol Rev 2014;34:645‐57. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Svaldi J, Schmitz F, Baur J et al. Efficacy of psychotherapies and pharmacotherapies for bulimia nervosa. Psychol Med 2019;49:898‐910. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Brownley KA, Berkman ND, Peat CM et al. Binge‐eating disorder in adults: a systematic review and meta‐analysis. Ann Intern Med 2016;165:409‐20. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Hilbert A, Petroff D, Herpertz S et al. Meta‐analysis of the efficacy of psychological and medical treatments for binge‐eating disorder. J Consult Clin Psychol 2019;87:91‐105. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. de Vos J, Houtzager L, Katsaragaki G et al. Meta analysis on the efficacy of pharmacotherapy versus placebo on anorexia nervosa. J Eat Disord 2014;2:27. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Hay PJ, Claudino AM, Touyz S et al. Individual psychological therapy in the outpatient treatment of adults with anorexia nervosa. Cochrane Database Syst Rev 2015;7:CD003909. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Solmi M, Wade TD, Byrne S et al. Comparative efficacy and acceptability of psychological interventions for the treatment of adult outpatients with anorexia nervosa: a systematic review and network meta‐analysis. Lancet Psychiatry 2021;8:215‐24. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. van den Berg E, Houtzager L, de Vos J et al. Meta‐analysis on the efficacy of psychological treatments for anorexia nervosa. Eur Eat Disord Rev 2019;27:331‐51. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Liang L, Huang Y, Xu R et al. Eszopiclone for the treatment of primary insomnia: a systematic review and meta‐analysis of double‐blind, randomized, placebo‐controlled trials. Sleep Med 2019;62:6‐13. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Kishi T, Nomura I, Matsuda Y et al. Lemborexant vs suvorexant for insomnia: a systematic review and network meta‐analysis. J Psychiatr Res 2020;128:68‐74. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta‐analysis. Sleep Med 2014;15:385‐92. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Winkler A, Auer C, Doering BK et al. Drug treatment of primary insomnia: a meta‐analysis of polysomnographic randomized controlled trials. CNS Drugs 2014;28:799‐816. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Kleinstauber M, Witthoft M, Steffanowski A et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev 2014;11:CD010628. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. van Dessel N, den Boeft M, van der Wouden JC et al. Non‐pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev 2014;11:CD011142. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Storebo OJ, Stoffers‐Winterling JM, Vollm BA et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev 2020;5:CD012955. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Cheng YC, Huang YC, Huang WL. Gabapentinoids for treatment of alcohol use disorder: a systematic review and meta‐analysis. Hum Psychopharmacol 2020;35:1‐11. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Donoghue K, Elzerbi C, Saunders R et al. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta‐analysis. Addiction 2015;110:920‐30. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Magill M, Ray L, Kiluk B et al. A meta‐analysis of cognitive‐behavioral therapy for alcohol or other drug use disorders: treatment efficacy by contrast condition. J Consult Clin Psychol 2019;87:1093‐105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Vanderkam P, Solinas M, Ingrand I et al. Effectiveness of drugs acting on adrenergic receptors in the treatment for tobacco or alcohol use disorders: systematic review and meta‐analysis. Addiction 2021;116:1011‐20. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Castells X, Blanco‐Silvente L, Cunill R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2018;8:CD007813. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Cunill R, Castells X, Tobias A et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta‐analysis and meta‐regression in over 9000 patients. Psychopharmacology 2016;233:187‐97. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Cortese S, Adamo N, Del Giovane C et al. Comparative efficacy and tolerability of medications for attention‐deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2018;5:727‐38. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Lenzi F, Cortese S, Harris J et al. Pharmacotherapy of emotional dysregulation in adults with ADHD: a systematic review and meta‐analysis. Neurosci Biobehav Rev 2018;84:359‐67. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Maneeton N, Maneeton B, Suttajit S et al. Exploratory meta‐analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther 2014;8:1685‐93. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Huhn M, Nikolakopoulou A, Schneider‐Thoma J et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi‐episode schizophrenia: a systematic review and network meta‐analysis. Lancet 2019;394:939‐51. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Hutton P, Taylor PJ, Mulligan L et al. Quetiapine immediate release v. placebo for schizophrenia: systematic review, meta‐analysis and reappraisal. Br J Psychiatry 2015;206:360‐70. [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. Kishi T, Ikuta T, Matsuda Y et al. Aripiprazole vs. brexpiprazole for acute schizophrenia: a systematic review and network meta‐analysis. Psychopharmacology 2020;237:1459‐70. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. McCutcheon RA, Pillinger T, Mizuno Y et al. The efficacy and heterogeneity of antipsychotic response in schizophrenia: a meta‐analysis. Mol Psychiatry 2021;26:1310‐20. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 107. Ostuzzi G, Bertolini F, Del Giovane C et al. Maintenance treatment with long‐acting injectable antipsychotics for people with nonaffective psychoses: a network meta‐analysis. Am J Psychiatry 2021;178:424‐36. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Zhao MJ, Qin B, Wang JB et al. Efficacy and acceptability of cariprazine in acute exacerbation of schizophrenia: meta‐analysis of randomized placebo‐controlled trials. J Clin Psychopharmacol 2018;38:55‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Zheng W, Cai DB, Yang XH et al. Short‐term efficacy and tolerability of lurasidone in the treatment of acute schizophrenia: a meta‐analysis of randomized controlled trials. J Psychiatr Res 2018;103:244‐51. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Cella M, Preti A, Edwards C et al. Cognitive remediation for negative symptoms of schizophrenia: a network meta‐analysis. Clin Psychol Rev 2017;52:43‐51. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Jauhar S, McKenna PJ, Radua J et al. Cognitive‐behavioural therapy for the symptoms of schizophrenia: systematic review and meta‐analysis with examination of potential bias. Br J Psychiatry 2014;204:20‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Lutgens D, Gariepy G, Malla A. Psychological and psychosocial interventions for negative symptoms in psychosis: systematic review and meta‐analysis. Br J Psychiatry 2017;210:324‐32. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Bahji A, Ermacora D, Stephenson C et al. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta‐analysis. J Affect Disord 2020;269:154‐84. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Kishi T, Ikuta T, Matsuda Y et al. Mood stabilizers and/or antipsychotics for bipolar disorder in the maintenance phase: a systematic review and network meta‐analysis of randomized controlled trials Mol Psychiatry (in press). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Li DJ, Tseng PT, Stubbs B et al. Efficacy, safety and tolerability of aripiprazole in bipolar disorder: an updated systematic review and meta‐analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry 2017;79:289‐301. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Lindstrom L, Lindstrom E, Nilsson M et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – A systematic review and meta‐analysis. J Affect Disord 2017;213:138‐50. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Liu B, Zhang Y, Fang H et al. Efficacy and safety of long‐term antidepressant treatment for bipolar disorders – A meta‐analysis of randomized controlled trials. J Affect Disord 2017;223:41‐8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Miura T, Noma H, Furukawa TA et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta‐analysis. Lancet Psychiatry 2014;1:351‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Pinto JV, Saraf G, Vigo D et al. Cariprazine in the treatment of bipolar disorder: a systematic review and meta‐analysis. Bipolar Disord 2020;22:360‐71. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Talaei A, Pourgholami M, Khatibi‐Moghadam H et al. Tamoxifen: a protein kinase C inhibitor to treat mania: a systematic review and meta‐analysis of randomized, placebo‐controlled trials. J Clin Psychopharmacol 2016;36:272‐5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 121. Oud M, Mayo‐Wilson E, Braidwood R et al. Psychological interventions for adults with bipolar disorder: systematic review and meta‐analysis. Br J Psychiatry 2016;208:213‐22. [ DOI ] [ PubMed ] [ Google Scholar ]
- 122. Amick HR, Gartlehner G, Gaynes BN et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta‐analysis. BMJ 2015;351:h6019. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 123. Cuijpers P, Karyotaki E, Andersson G et al. The effects of blinding on the outcomes of psychotherapy and pharmacotherapy for adult depression: a meta‐analysis. Eur Psychiatry 2015;30:685‐93. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Cuijpers P, Donker T, Weissman MM et al. Interpersonal psychotherapy for mental health problems: a comprehensive meta‐analysis. Am J Psychiatry 2016;173:680‐7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 125. Imai H, Tajika A, Chen P et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev 2016;10: CD011170. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 126. Merz J, Schwarzer G, Gerger H. Comparative efficacy and acceptability of pharmacological, psychotherapeutic, and combination treatments in adults with posttraumatic stress disorder: a network meta‐analysis. JAMA Psychiatry 2019;76:904‐13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 127. Sonis J, Cook JM. Medication versus trauma‐focused psychotherapy for adults with posttraumatic stress disorder: a systematic review and meta‐analysis. Psychiatry Res 2019;282:112637. [ DOI ] [ PubMed ] [ Google Scholar ]
- 128. Burkner PC, Bittner N, Holling H et al. D‐cycloserine augmentation of behavior therapy for anxiety and obsessive‐compulsive disorders: a meta‐analysis. PLoS One 2017;12:e0173660. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 129. Cuijpers P, Sijbrandij M, Koole SL et al. Adding psychotherapy to antidepressant medication in depression and anxiety disorders: a meta‐analysis. World Psychiatry 2014;13:56‐67. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 130. Driessen E, Dekker JJM, Peen J et al. The efficacy of adding short‐term psychodynamic psychotherapy to antidepressants in the treatment of depression: a systematic review and meta‐analysis of individual participant data. Clin Psychol Rev 2020;80:101886. [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Karyotaki E, Smit Y, Holdt Henningsen K et al. Combining pharmacotherapy and psychotherapy or monotherapy for major depression? A meta‐analysis on the long‐term effects. J Affect Disord 2016;194:144‐52. [ DOI ] [ PubMed ] [ Google Scholar ]
- 132. Ori R, Amos T, Bergman H et al. Augmentation of cognitive and behavioural therapies (CBT) with d‐cycloserine for anxiety and related disorders. Cochrane Database Syst Rev 2015;5:CD007803. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 133. Hoskins MD, Sinnerton R, Nakamura A et al. Pharmacological‐assisted psychotherapy for post‐traumatic stress disorder: a systematic review and meta‐analysis. Eur J Psychotraumatol 2021;12:1853379. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 134. Lopez PL, Torrente FM, Ciapponi A et al. Cognitive‐behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev 2018;3:CD010840. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 135. Kolovos S, van Tulder MW, Cuijpers P et al. The effect of treatment as usual on major depressive disorder: a meta‐analysis. J Affect Disord 2017;210:72‐81. [ DOI ] [ PubMed ] [ Google Scholar ]
- 136. Stoffers JM, Lieb K. Pharmacotherapy for borderline personality disorder – current evidence and recent trends. Curr Psychiatry Rep 2015;17:534. [ DOI ] [ PubMed ] [ Google Scholar ]
- 137. Sakaluk JK, Kilshaw RE, Williams AJ et al. Evaluating the evidential value of empirically supported psychological treatments (ESTs): a meta‐scientific review. J Abnorm Psychol 2019;128:500‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 138. Jauhar S, Laws KR, McKenna PJ. CBT for schizophrenia: a critical viewpoint. Psychol Med 2019; 49:1233‐6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 139. Bighelli I, Huhn M, Schneider‐Thoma J et al. Response rates in patients with schizophrenia and positive symptoms receiving cognitive behavioural therapy: a systematic review and single‐group meta‐analysis. BMC Psychiatry 2018;18:380. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 140. Leucht S, Hierl S, Kissling W et al. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta‐analyses. Br J Psychiatry 2012;200:97‐106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 141. Faltinsen EG, Storebo OJ, Jakobsen JC et al. Network meta‐analysis: the highest level of medical evidence? BMJ Evid Based Med 2018;23:56‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 142. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 143. Leucht S, Chaimani A, Cipriani AS et al. Network meta‐analyses should be the highest level of evidence in treatment guidelines. Eur Arch Psychiatry Clin Neurosci 2016;266:477‐80. [ DOI ] [ PubMed ] [ Google Scholar ]
- 144. Cipriani A, Higgins JP, Geddes JR et al. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med 2013;159:130‐7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 145. Veroniki AA, Mavridis D, Higgins JP et al. Characteristics of a loop of evidence that affect detection and estimation of inconsistency: a simulation study. BMC Med Res Methodol 2014;14:106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407‐15. [ DOI ] [ PubMed ] [ Google Scholar ]
- 147. Leucht S, Fennema H, Engel R et al. What does the HAMD mean? J Affect Disord 2013;148:243‐8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Moncrieff J, Kirsch I. Empirically derived criteria cast doubt on the clinical significance of antidepressant‐placebo differences. Contemp Clin Trials 2015;43:60‐2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. Leucht S, Kane JM, Etschel E et al. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 2006;31:2318‐25. [ DOI ] [ PubMed ] [ Google Scholar ]
- 150. Man‐Son‐Hing M, Laupacis A, O'Rourke K et al. Determination of the clinical importance of study results. J Gen Intern Med 2002;17:469‐76. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 151. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA 2014;312:1342‐3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Hengartner MP, Ploderl M. Statistically significant antidepressant‐placebo differences on subjective symptom‐rating scales do not prove that the drugs work: effect size and method bias matter! Front Psychiatry 2018;9:517. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 153. Cuijpers P, Quero S, Papola D et al. Care‐as‐usual control groups across different settings in randomized trials on psychotherapy for adult depression: a meta‐analysis. Psychol Med 2021;51:634‐44. [ DOI ] [ PubMed ] [ Google Scholar ]
- 154. Baskin TW, Tierney SC, Minami T et al. Establishing specificity in psychotherapy: a meta‐analysis of structural equivalence of placebo controls. J Consult Clin Psychol 2003;71:973‐9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 155. Sherman RE, Anderson SA, Dal Pan GJ et al. Real‐world evidence – What is it and what can it tell us? N Engl J Med 2016;375:2293‐7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 156. Cuijpers P, Karyotaki E, Reijnders M et al. Is psychotherapy effective? Pretending everything is fine will not help the field forward. Epidemiol Psychiatr Sci 2019;28:356‐7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 157. Nicholson JM, Ioannidis JP. Research grants: conform and be funded. Nature 2012;492:34‐6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 158. Open Science Collaboration. Estimating the reproducibility of psychological science. Science 2015;349:aac4716. [ DOI ] [ PubMed ] [ Google Scholar ]
- 159. Munafò MR, Nosek BA, Bishop DV et al. A manifesto for reproducible science. Nat Hum Behav 2017;1:0021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 160. Maj M, Stein DJ, Parker G et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020;19:269‐93. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 161. Maj M, van Os J, De Hert M et al. the clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021;20:4‐33. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 162. Stein DJ, Craske MG, Rothbaum BO et al. The clinical characterization of the adult patient with an anxiety or related disorder aimed at personalization of management. World Psychiatry 2021;20:336‐56. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 163. Cuijpers P. Targets and outcomes of psychotherapies for mental disorders: an overview. World Psychiatry 2019;18:276‐85. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 164. Fava G, Tomba E, Sonino N. Clinimetrics: the science of clinical measurements. Int J Clin Pract 2012;66:11‐5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 165. Steinert C, Kruse J, Leichsenring F. Long‐term outcome and non‐response in psychotherapy: are we short‐sighted. Psychother Psychosom 2016;85:235‐7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 166. Markowitz JC, Milrod BL. What to do when a psychotherapy fails. Lancet Psychiatry 2015;2:186‐90. [ DOI ] [ PubMed ] [ Google Scholar ]
- 167. Nutt D. Help luck along to find psychiatric medicines. Nature 2014;515:165. [ DOI ] [ PubMed ] [ Google Scholar ]
- 168. Barlow J, Schrader McMillan A, Kirkpatrick S et al. Health‐led interventions in the early years to enhance infant and maternal mental health: a review of reviews. Child Adolesc Ment Health 2010;15:178‐85. [ DOI ] [ PubMed ] [ Google Scholar ]
- 169. Fusar‐Poli P, Correll CU, Arango C et al. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry 2021:20:200‐21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- Collections

Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. York (UK): Centre for Reviews and Dissemination (UK); 1995-.

Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet].
Comparing the effectiveness of individual therapy and group therapy in the treatment of depression: systematic review.
B Hodgkinson , D Evans , A O'Donnell , and K Walsh .
Review published: 1999 .
- Authors' objectives
To present the best available information on the use of group therapy and individual therapy in the treatment of long term depression. The primary question to be addressed was: for the treatment of long term depression, is group therapy or individual therapy appropriate and, if so, in what form? If neither is indicated, what modality might be better?
CINAHL, MEDLINE, PsycLIT, Current Contents, Science Citation Index, the Cochrane Library, DARE and EMBASE were searched (search terms and dates not provided). Bibliographies of all identified studies and review papers were also searched. Dissertation Abstracts International and proceedings databases were searched for unpublished studies.
- Study selection
Study designs of evaluations included in the review
Randomised or pseudo-randomised controlled trials (RCTs).
Specific interventions included in the review
Various forms of individual therapy (defined as any one to one interaction between the patient and the therapist) and group therapy (excluding family therapy). Studies involving only combined psychotherapy and pharmacotherapy and studies which involved pharmacotherapy alone were excluded, as were treatments combining both group and individual therapy.
Participants included in the review
Children or adults suffering from long term depression and a determined Beck Depression Inventory (BDI) value of 12 or above or a Hamilton Rating Score for Depression (HRSD) of 14 or above were examined. Studies having participants with accompanying psychiatric disorders (e.g. schizophrenia) were excluded.
Outcomes assessed in the review
Reduction in depression inventory scores such as BDI, HRSD.
How were decisions on the relevance of primary studies made?
Two reviewers assessed all identified abstracts for relevance. Studies identified from bibliography searches were assessed on study title.
- Assessment of study quality
Methodological quality was assessed using a checklist that was designed and trialed by the Joanna Briggs Institute for Evidence Based Nursing and Midwifery (JBIEBNM) and criteria included: randomisation, blinding, intention-to-treat analysis, comparability of groups at baseline, identical treatment of groups other than for named interventions, outcome assessment, statistical analysis. At least two reviewers were involved in assessing validity as the authors state that disagreements between reviewers were resolved by discussion with another reviewer.
- Data extraction
Data were extracted independently using a data extraction tool that was developed and tested prior to use by JBIEBNM. A separate reviewer dealt with disagreements.
- Methods of synthesis
How were the studies combined?
If appropriate with available data, results from comparable groups of studies were pooled by meta-analysis. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for categorical data, and weighted mean differences (WMD) and 95% CIs for continuous data. Where possible intention-to-treat and/or completer analysis was performed. Where statistical pooling was not appropriate or possible, the findings were summarised in narrative form.
How were differences between studies investigated?
Heterogeneity between combined studies was tested using a standard chi-squared test.
- Results of the review
Nineteen RCTs and one systematic review. Numbers of participants were not clear.
Individual cognitive behavioural therapy versus pharmacotherapy (3 studies): both treatments were found to be effective in reducing BDI scores (n=155; WMD 1.3 favouring pharmacotherapy (95% CI: -1.3, 4.0)) and HRSD scores (n=138; WMD -0.6 favouring cognitive therapy (95% CI: -2.5, 1.2)). The authors note that the included studies predate selective serotonin reuptake inhibitors (SSRIs).
Individual cognitive therapy versus individual cognitive therapy combined with pharmacotherapy (2 studies): neither ICT nor combined therapy was found to be significantly more effective than the other in either trial. Due to the nature of the reporting of data, meta-analysis was not possible.
Individual cognitive therapy versus waiting list with medication support (1 study): ICT significantly reduced BDI scores over the period of treatment and at 6 month follow up compared to the control (p<0.05).
Cognitive individual and group therapy versus waiting list with support (treatment as usual) (2 studies): One study found significant reductions in BDI scores for group cognitive therapy compared to control at post treatment but the groups were not compared at follow-up. The other study found significant reductions in BDI scores for individual therapy compared to control at post treatment but not at 3 months follow-up. The two studies could not be combined in a meta-analysis.
Cognitive individual or group therapy versus waiting list (no other treatment) (2 studies): In one study group therapy generated significantly lower BDI and HRSD scores than control. In the other study, individual therapy was significantly more effective than control in reducing BDI and HRSD scores at post-treatment and at 2 month follow-up. Meta-analysis of these two studies could not be performed.
Cognitive group therapy versus individual cognitive therapy (4 studies): BDI scores post treatment (n=124) WMD 0.2 (95% CI: -2.1, 2.6) and HRSD scores (2 studies, n=42)) WMD -0.3 (95% CI: -3.6, 3.0). Follow up BDI scores were not significantly different between groups at 2 and 3 months, but at 6 months CGT was favoured (n=62; WMD -6.9, 95% CI -11.6, -2.2). One study using ITT analysis claimed a significant advantage of ICT over CGT in BDI scores at post treatment and follow-up (p<0.02) and two did not.
Individual psychotherapy versus individual cognitive therapy (1 study): no significant difference between treatments was shown.
Cognitive group therapy versus computer assisted therapy (therapeutic learning programme or TLP) (2 studies): both studies reported no significant difference in the effectiveness of treatments in reducing depression scores; meta-analysis could not be performed.
Coping with depression: a course, comparison of group or class therapy and individual therapy (2 studies): Meta-analysis showed individual treatment was significantly more effective at reducing BDI scores than was the class method (n=104; WMD 3.0, 95% CI 1.0, 5.0), however the effect did not persist at follow up at 1 and 6 months.
Individual cognitive therapy versus waiting list control (1 systematic review): Remission from depressive disorder was higher in the ICT group compared to control OR 2.2 (95% CI: 1.4, 3.5).
Cognitive group therapy versus waiting list control (2 studies): CGT was significantly better than control in reducing BDI scores post-treatment (n=56; WMD -11.2, 95% CI -16, -6.5). One study found this difference persisted at follow-up. CGT was also significantly better than control for producing participants with normal BDI scores post-treatment (n=46; Peto OR 11.8, 95% CI 3.3, 42.3).
- Authors' conclusions
Individual and group cognitive behavioural therapies for moderately or severely depressed adults (BDI 14 or above) are comparable with each other in effectiveness and both are superior to providing no treatment at all. Individual cognitive therapy is equal to or better than tricyclic antidepressant drugs given at recommended therapeutic dosages for depressed people with a mean BDI of 30. This information is based on level II evidence (RCT).
- CRD commentary
This is a good review. The inclusion criteria are clearly defined relative to the research question and the literature search is very comprehensive so it is unlikely that any studies would have been missed, although it is not stated whether studies were restricted by language. Details of the review process are given and validity was assessed but not used. Study details were not fully presented and could have been presented in an appendix. It may have been more appropriate to use relative risk rather than odds ratio as a summary estimate. Each comparison is based on quite a small number of participants in a few trials, and some reported only pre- versus post-treatment data rather than results of a randomised comparison. The authors' conclusions do follow from the results but should be treated with some caution owing to flaws in methodological quality of the included trials reported in the text.
- Implications of the review for practice and research
Practice: The authors state practice implications for adults and adolescents.
For adults, either group (CGT) or individual cognitive behavioural therapy (ICT) can be used to treat moderate to severely depressed patients with the choice of therapy dependent upon the clinicians perceived receptiveness of the individual patient to group versus individual treatment. The use of computer assisted therapy can be useful as an aid to CGT in moderate to severely depressed patients. ICT can be effective in place of pharmacotherapy in moderate to severely depressed patients if the patient is opposed to being treated with drug therapy. CGT has not been compared to pharmacotherapy so no direct recommendation can be given as to its effectiveness as a replacement therapy.
For adolescents, either group or individual cognitive behavioural therapy can be used to treat moderately depressed adolescents (BDI 14 or above).
Research: The authors state that more research is needed to determine the effectiveness of individual or group cognitive behavioural therapy in severely depressed adolescents (BDI 20 or above).
- Bibliographic details
Hodgkinson B, Evans D, O'Donnell A, Walsh K. Comparing the effectiveness of individual therapy and group therapy in the treatment of depression: systematic review. Adelaide, S. Australia, Australia: Joanna Briggs Institute for Evidence Based Nursing and Midwifery. Systematic Review; 3. 1999.
- Indexing Status
Subject indexing assigned by CRD
Cognitive Therapy; Depressive Disorder /therapy
- AccessionNumber
11999009777
- Database entry date
- Record Status
This is a critical abstract of a systematic review that meets the criteria for inclusion on DARE. Each critical abstract contains a brief summary of the review methods, results and conclusions followed by a detailed critical assessment on the reliability of the review and the conclusions drawn.
- Cite this Page Hodgkinson B, Evans D, O'Donnell A, et al. Comparing the effectiveness of individual therapy and group therapy in the treatment of depression: systematic review. 1999. In: Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. York (UK): Centre for Reviews and Dissemination (UK); 1995-.
In this Page
Recent activity.
- Comparing the effectiveness of individual therapy and group therapy in the treat... Comparing the effectiveness of individual therapy and group therapy in the treatment of depression: systematic review - Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
The Effectiveness of Psychological Interventions Delivered in Routine Practice: Systematic Review and Meta-analysis
- Original Article
- Open access
- Published: 06 October 2022
- Volume 50 , pages 43–57, ( 2023 )
Cite this article
You have full access to this open access article

- Chris Gaskell ORCID: orcid.org/0000-0002-7589-5246 1 ,
- Melanie Simmonds-Buckley ORCID: orcid.org/0000-0003-3808-4134 1 ,
- Stephen Kellett ORCID: orcid.org/0000-0001-6034-4495 1 , 2 ,
- C. Stockton 1 ,
- Erin Somerville 1 ,
- Emily Rogerson 1 &
- Jaime Delgadillo ORCID: orcid.org/0000-0001-5349-230X 1
9410 Accesses
13 Citations
111 Altmetric
12 Mentions
Explore all metrics
This review presents a comprehensive evaluation of the effectiveness of routinely delivered psychological therapies across inpatient, outpatient and University-based clinics. This was a pre-registered systematic-review of studies meeting pre-specified inclusion criteria (CRD42020175235). Eligible studies were searched in three databases: MEDLINE, CINAHL and PsycInfo. Pre–post treatment (uncontrolled) effect sizes were calculated and pooled using random effects meta-analysis to generate effectiveness benchmarks. Moderator analyses were used to examine sources of heterogeneity in effect sizes. Overall, 252 studies ( k = 298 samples) were identified, of which 223 ( k = 263 samples) provided sufficient data for inclusion in meta-analysis. Results showed large pre–post treatment effects for depression [ d = 0.96, (CI 0.88–1.04), p ≤ 0.001, k = 122], anxiety [ d = 0.8 (CI 0.71–0.9), p ≤ 0.001, k = 69], and other outcomes [ d = 1.01 (CI 0.93–1.09), p ≤ 0.001, k = 158]. This review provides support for the effectiveness of routinely delivered psychological therapy. Effectiveness benchmarks are supplied to support service evaluations across multiple settings.
Similar content being viewed by others

Psychological interventions for people with psychotic experiences: protocol for a systematic review and meta-analysis

A transdiagnostic meta-analysis of acute augmentations to psychological therapy
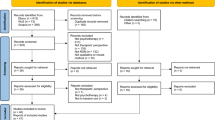
Psychotherapists’ Experience with In-Session Use of Routine Outcome Monitoring: A Qualitative Meta-analysis
Avoid common mistakes on your manuscript.
Introduction
Meta-analyses of clinical trials support the efficacy of psychological interventions for various mental health problems such as depression (Cuijpers et al., 2008 ), anxiety disorders (e.g., Cuijpers et al., 2014a ; Mayo-Wilson et al., 2014 ; Olatunji et al., 2014 ; Sánchez-Meca et al., 2010 ; Wolitzky-Taylor et al., 2008 ), post-traumatic stress disorder (Lewis et al., 2020 ), obsessive–compulsive disorder (Rosa-Alcázar et al., 2008 ), eating disorders (Linardon et al., 2017 ) and other conditions. Grounded in this evidence, clinical guidelines support the use of psychological interventions in routine clinical care (e.g., Chambless & Hollon, 1998 ; Chambless & Ollendick, 2001 ; National Institute for Health & Care Excellence, 2011 ). These guidelines commonly advocate the implementation of empirically supported treatments, closely following the procedures implemented in clinical trials and specified in associated treatment manuals. To this end, competency frameworks have been developed to support the dissemination of empirically supported treatments in routine care and clinical training programmes (e.g., Lemma et al., 2008 ; Roth & Pilling, 2008 ; Roth et al., 2009 ).
Some studies have found similar treatment outcomes when comparing data from efficacy trials and routine practice (e.g., Lutz et al., 2016 ; Persons et al., 1999 ). However, there are some reasons to assume that the effects of psychotherapy delivered in routine care settings may differ from those observed in clinical trials. Recent evidence indicates that psychological treatment outcomes are associated with treatment integrity , which refers to the competent (skilled) delivery of protocol-driven treatment procedures (Power et al., 2022 ). However, surveys of clinicians working in routine settings often reveal negative attitudes towards protocol-driven treatment and a lack of adherence to treatment manuals (e.g., Addis & Krasnow, 2000 ). Hence, the integrity of routinely delivered psychological treatments is unclear, and it probably varies across services (Freedland et al., 2011 ). Furthermore, the strict selection criteria applied in clinical trials may result in unusually homogeneous samples that do not reflect the diverse clinical populations typical of routine care settings (e.g., Lambert, 2013 ; Zimmerman et al., 2002 ). Previous studies have found systematic differences in the clinical profiles of patients included and excluded from psychotherapy trials (e.g., van der Lem et al., 2012 ). For these reasons, it is plausible to assume that the effects of routinely delivered therapy may vary across settings and clinical populations, and may not necessarily conform to benchmarks from efficacy trials.
A tradition of practice-based evidence (PBE, Margison et al., 2000 ) has emerged in recent decades, with numerous studies examining the effects of routinely delivered psychological interventions in various settings. Narrative reviews of PBE generally confirm that moderate-to-large uncontrolled (pre-to-post treatment) effect sizes are observed in routine care settings, supporting the effectiveness of psychotherapy but also demonstrating considerable variability across patient samples, therapists and clinics (e.g., see Barkham et al., 2010 ; Castonguay et al., 2013 , 2021 ). An inherent limitation of such narrative reviews is that they perform a selective rather than systematic synthesis of available data. Benchmarking studies can be useful to provide general indices of treatment effectiveness, enabling services to evaluate their outcomes relative to efficacy trials (e.g., McAleavey et al., 2019 ; Minami et al., 2008 ) or aggregated effect size data from similar clinical services (e.g., Delgadillo et al., 2014 ). Psychotherapy benchmarking studies tend to report favorable pooled effects sizes, but also show variability in effects across clinics (e.g., Barkham et al., 2001 ; Connell et al., 2007 ; Delgadillo et al., 2014 ; Gyani et al., 2013 ). Although benchmarking studies help to quantify the expected magnitude of treatment effects observed in routine clinical settings, most are nevertheless circumscribed to small sets of clinics or geographical areas, offering limited insights into possible sources of heterogeneity in treatment outcomes. Systematic reviews and meta-analyses may therefore offer a more comprehensive examination of the effectiveness of routinely delivered treatments.
Some meta-analytic investigations have reported that outcomes from routine practice-based treatments are not as favorable as those delivered in research settings (Weisz et al., 1995 ). Other meta-analyses suggest that there are no differences in treatment effects when comparing PBE and efficacy studies after controlling for case-mix differences (e.g., Shadish et al., 1997 , 2000 ). However, many of the PBE studies in these meta-analyses applied stringent controls on the treatment procedures (e.g., adherence and competence assessments)—making them more akin to efficacy trials. Hunsley and Lee ( 2007 ) reviewed 35 studies and concluded that the completion and improvement rates observed in PBE studies were comparable to efficacy trials. Cahill et al. ( 2010 ) reviewed 31 studies, concluding that psychotherapy was most effective for the treatment of common mental disorders, with a pooled uncontrolled effect size of d = 1.29. More recently, Wakefield et al. ( 2021 ) reviewed 60 studies, of which 47 were eligible for meta-analysis. They reported large uncontrolled effect sizes for depression ( d = 0.87) and anxiety ( d = 0.88), and a moderate effect on functional impairment ( d = 0.55). These meta-analyses show wide variability in treatment effects (i.e., heterogeneity) across studies/samples.
PBE meta-analyses provide some insights into plausible sources of heterogeneity, including methodological (e.g., completers analyses vs. inclusion of patients lost to follow-up) and clinical features (e.g., larger effects for common mental disorders, lower effects for patients with comorbidities and socioeconomic disadvantages, larger effects for lengthier interventions). Nevertheless, these meta-analyses are over a decade old (Cahill et al., 2010 ; Hunsley & Lee, 2007 ) or limited to a specific treatment setting (e.g., primary care outpatient services; Wakefield et al., 2021 ). Further research into the methodological and clinical sources of treatment heterogeneity is needed to better understand why treatment effects vary across samples, and to determine whether or not these effects vary across different treatment settings (e.g., outpatient, inpatient, university-based treatment).
The considerable growth of the PBE literature in the last decade and Implementation of empirically supported treatments across many settings warrants a comprehensive review of treatment outcomes data. The aim of the present study was to systematically review available PBE studies. The objectives of the study were to (1) provide benchmarks of treatment effectiveness using meta-analysis and (2) to examine sources of effect size heterogeneity using pre-specified moderator analyses informed by earlier studies.
Search Strategy and Study Selection
The present study followed good practice guidelines for systematic reviews (PRISMA, Page et al., 2021 ) and meta-analyses of psychotherapy studies (MAP-24, Flückiger et al., 2018 ). A review protocol was pre-registered in the PROSPERO database (CRD42020175235). Footnote 1 Literature searches were carried out without any restrictions on date of publication up to the search date (April 2020). Inclusion criteria were: (a) studies reporting outcomes for routinely delivered treatments (i.e., not as part of efficacy trials); (b) all adult sample (no patients under 16); (c) employed a psychological treatment (i.e., driven by psychological theory and intended to be therapeutic (Spielmans & Flückiger, 2018 ), as inferred or described by study manuscripts); and (d) conducted face-to-face. Studies were excluded if they: used (e) family/group treatments, (f) were not available in English; (g) did not employ a self-report measure of treatment effectiveness Footnote 2 ; (h) did not provide sufficient data to calculate pre–post treatment effect sizes; or (i) employed randomization procedures or control groups. A more detailed table of inclusion/exclusion criteria is available in supplementary Table 1.
The search strategy had three phases. Phase one was a systematic search of three electronic literature databases (MEDLINE, CINAHL and PsycInfo) via EBSCO using a pre-registered list of key terms. Methodological terms included: practice-based evidence , routine practice , benchmarking , transportability , transferability , clinically representative , managed care setting , uncontrolled , external validity , applicable findings , empirically supported , dissemination , and clinical effectiveness evaluation . These terms were informed by prior reviews of psychotherapy effectiveness (Cahill et al., 2010 ; Stewart & Chambless, 2009 ). Effectiveness and evaluation were not used as single word terms due to producing unmanageable numbers of irrelevant records. For the psychologically relevant term: psycho * OR therap * was used for PsycInfo while psycho * alone was used for MEDLINE and CINAHL ( therap * was removed from MEDLINE/CINAHL due to producing an unmanageable number of irrelevant records). Limiters included adult population and English language . No exclusions were made based on the type of publication. Key term combinations and Boolean operators are reported in supplementary Table 2. Phase two included a manual search of reference lists, and forward citation searching (using Google Scholar) for studies identified in phase one. Titles relevant to the current review were identified by the first author. Finally, phase three was a grey literature search using the terms psychotherapy AND routine-practice AND effectiveness in Google Scholar.
After removal of duplicates, titles and abstracts of potentially eligible studies were screened by the first author using a pre-developed and piloted screening tool. Sub-samples were screened by a second coder at each stage (20% at the stage of title screening; 10% at the stage of full-text screening). Percentage agreement and inter-rater reliability statistics (Kappa ( \(\kappa\) ), Cohen, 1960 ] indicated good reliability ( \(\kappa\) = 0.78, 1713/1740, 98.45%) in the first stage and adequate reliability ( \(\kappa\) = 0.65, 24/30, 80%) in the second stage. After the selection process was completed, corresponding authors for eligible studies were contacted via email to request additional recommendations for potentially eligible studies, and to request additional statistical information to calculate effect sizes. E-mail responses were received from 76 authors and additional data was provided for 41 samples.
Data Extraction
There were three separate outcome domains (and subsequently three meta-analyses) for ‘depression’, ‘anxiety’ and ‘other’ outcomes. The latter category consisted of general psychological distress scales, measures of functioning/quality of life, or diagnosis-specific outcome scales (e.g., obsessive-compulsive disorder, etc.). A pilot extraction sheet was developed and pilot-tested with a sample of studies ( k = 10). When multiple samples were reported in the same study, effect-sizes across these samples were aggregated to reduce bias of statistical dependency (Gleser & Olkin, 2009 ; Hoyt & Del Re, 2018 ). To avoid loss of information (e.g., aggregating sub-samples that are distinct based on levels of a moderator), study samples were disaggregated for moderator analyses (Cooper, 1998 ). Studies with overlapping datasets (e.g., reanalysis of the same sample) were only included once in the meta-analysis. Samples which performed an intention-to-treat (ITT) analysis were preferred to completer samples due to being less prone to attrition bias (Jüni et al., 2001 ); so the ITT data was extracted for studies that reported both ITT and completer analyses. As extraction of multiple study effect-sizes within a single domain (e.g., depression) threatens statistical dependency (Borenstein et al., 2021 ) we selected a single effect-size per domain (Card, 2015 ; Cuijpers, 2016 ), using a preference system (defined a priori, supplementary material). Reliability of coding for effect-size data was computed using a second coder for a sub-sample of manuscripts (n = 29) demonstrating almost perfect reliability across all values ( \(\kappa\) = 0.97, agreement = 97.56%) and perfect reliability for effect-size values ( \(\kappa\) = 1.00). Key categorical and numerical variables extracted from manuscripts for moderator analyses are reported in Table 1 . For sample severity, the decision was made to cluster university counselling centers in the ‘mild’ severity category due to prior research finding normative data of UK University students comparable to primary care samples (Connell et al., 2007 ).
Risk of Bias and Quality Assessment
The Joanna Briggs Institute Quality Appraisal Tool for Case Series (Munn et al., 2020 ) was used to assess risk of bias. Eight criteria primarily focusing upon manuscript reporting detail were used. Criteria included manuscript reporting of: (i) patient inclusion criteria, (ii) service description, (iii) treatment description, (iv) sample characteristics, (v) outcome data, (vi) effect-size calculation, (vii) consecutive patient recruitment, and (viii) inclusion of patients lost to follow-up in statistical analysis. Each item was coded as either met or not met (including not clear) by the first author for each sample. A sub-sample (23.8%) was rated independently by two other reviewers (11.9% each). The pooled agreement was 84.17% ( \(\kappa\) = 0.62).
Statistical Analysis
All analyses were conducted using the R statistical analysis environment (R Core Team, 2020 , v 4.0.2). We calculated standardised mean change (SMC: Becker, 1988 ) for included studies using the metafor package. This approach divides the pre–post mean change score by the pretreatment standard deviation with a sampling variance adjustment using the correlation between the pre-treatment and post-treatment measures (Morris, 2008 ). When unavailable, Pearson’s r was imputed using an empirically derived estimate ( r = .60, Balk et al., 2012 ). Aggregation of samples/sampling errors was conducted using the aggregate function of metafor using standard inverse-variance weighting.
Random effects meta-analyses were performed using the metafor (Viechtbauer, 2020 ), dmetar (Harrer et al., 2019 ), and meta (Schwarzer, 2020 ) packages. Forest plots were used to visualise pre–post treatment effects sizes across samples. Effect size heterogeneity was assessed using I 2 (Higgins & Thompson, 2002 ) and the Q statistic (Cochran, 1954 ). Publication bias was examined using funnel plots and assessed statistically using rank correlation tests (Begg & Mazumdar, 1994 ), Egger’s regression test for funnel plot asymmetry (Egger et al., 1997 ), and the fail-safe N (Rosenthal method, Rosenthal, 1979 ).
Moderator analyses were based on a set of moderator variables selected a priori, following evidence from prior reviews. Subgroup variables included: (i) analysis (inclusion of patients lost to follow-up), (ii) geographical region , (iii) severity (mild, moderate, severe, university Footnote 3 ), (iv) treatment modality , (v) experience [unqualified (i.e., trainees) vs. qualified therapists], (vi) stage of treatment development (preliminary study vs. routine evaluations), and (vii) sample size (small, medium, large). Continuous meta-regression variables included (i) publication year , (ii) average age of sample, and (iii) percentage of samples who identified as female . All moderators were included in meta-regression which was based on a mixed effects (i.e., multilevel) model (Borenstein et al., 2021 ) with weighted estimation (inverse-variance weights).
Finally, we developed effect size benchmarks to support the evaluation of effectiveness across four broad settings: outpatient services, inpatient services and university counselling services (i.e., student population) and university psychotherapy clinics (non-student population). Informed by previous benchmarking studies (Delgadillo et al., 2014 ), pooled effect sizes (using random effects meta-analyses) were stratified into quartiles to differentiate between low effectiveness (bottom 25%), average effectiveness (middle 50%) and high effectiveness benchmarks (top 25%).
Search Results
The PRISMA diagram in Fig. 1 presents a summary of the study selection process. Overall, 10,503 records were identified, of which 252 manuscripts were eligible for inclusion and 223 (samples k = 263) had sufficient information to be included in the meta-analysis. Summary statistics are provided in Table 2 .

Prisma flow diagram of studies throughout the review
Study Characteristics
Eligible studies were published between 1984 and 2020 (median = 2013, k = 294 published ≥ 2000). Of these, 169 samples included patients lost to follow-up ( k = 118, 56.72% completers). Most studies were from the USA ( k = 113, 37.92%), England ( k = 78, 26.17%), Germany ( k = 24, 8.05%), Sweden ( k = 12, 4.02%) and Canada ( k = 10, 3.36%). These five most represented countries accounted for most of the included samples ( k = 237, 79.53%).
Sample Characteristics
Sample characteristics were reported for 291 samples, with a cumulative N of 233,140 patients (mean = 838.63, median = 81.5, range—4 to 33,243, IQR = 224.5). The prevalence of female participants was 61.88% (N = 144,273, k = 279) with 13 all-female samples and 2 all-male samples. The mean average sample age was 35.33 years (range = 19.00–60.50). Across studies which provided information, 23.00% of patients were from ethnic minorities ( k = 127), 37.00% were married ( k = 106), and 23.00% were in employment ( k = 96).
Treatment Characteristics
Most samples evaluated cognitive-behavioral interventions ( k = 152, 51.01%) while 50 samples evaluated psychodynamic (16.78%), and 25 samples evaluated counselling (8.29%; other = 71, 23.82%). Counselling interventions were interventions described simply as ‘counselling’ by study authors (with no further treatment information) or ‘person-centered counselling’ interventions. Interventions termed ‘counselling’ but described in a way that fit closely with of the other treatment modalities (e.g., cognitive-behavioral counselling) was assigned to the more specific treatment modality group. For symptom severity, 96 (32.21%) samples came from services treating mild conditions, 92 (30.87%) from services treating moderate conditions, 33 (11.07%) from services treating severe conditions, and 68 (22.82%) from university psychotherapy clinics (not counselling centers) that treated a wide spectrum of conditions from mild-to-severe (other, k = 9, 3.02%). Treatment dosage, when reported ( k = 256) was in hours/sessions ( k = 225), months ( k = 12) or days ( k = 8). The pooled (non-weighted) average dose (hours) was 16.30 sessions (median = 13.00, range = 1.00–139.30, IQR = 11.00). A total of 62 (20.81%) samples reported that treatment was delivered exclusively by trainees, while 100 (35.58%) samples reported having at least one trainee.
Risk of Bias
In order of satisfactory criteria (e.g., the criterion under evaluation was met), the following risk of bias domains were assessed: demographic reporting detail (264/298, agreement = 98.33%, \(\kappa\) = 0.88), service reporting detail (260/298, agreement = 85%, \(\kappa\) = 0.31), study outcome reporting details (240/298, agreement = 83.33%, \(\kappa\) = − 0.03), intervention reporting detail (234/298, agreement = 85%, \(\kappa\) = 0.32), service inclusion criteria (214/298, agreement = 90%, \(\kappa\) = 0.64), appropriate use of analysis (214/298, agreement = 70%, \(\kappa\) = 0.26), complete inclusion (i.e. consecutive recruitment and inclusion of those lost to follow-up, 41/298, agreement = 85%, \(\kappa\) = 0.45), and consecutive inclusion (93/298, agreement = 76.67%, \(\kappa\) = 0.51).
Meta-analyses
The random-effects meta-analysis for depression outcomes ( k = 140, N = 68,077), across 10 unique measurement tools was statistically significant ( p ≤ 0.001), indicative of a large pre–post treatment ( d = 0.96, CI 0.88–1.04) reduction in depression severity. There was a large magnitude of statistically significant heterogeneity [I 2 = 97.94%, Q(df = 121) = 2677.37, p ≤ 0.001]. The funnel plot (Fig. 2 ) shows limited visual evidence of asymmetry. The funnel rank correlation test was not statistically significant ( \(\tau\) = 0.061, p = 0.46) however the funnel regression test was statistically significant (Z = 2.13, p = 0.033). The fail-safe N was 515,853.

Funnel plots displaying the distribution of studies reporting pre–post outcomes for (i) depression, (ii) anxiety, and (iii) miscellaneous outcomes
The random-effects meta-analysis for anxiety outcomes ( k = 84, N = 26,689, measurement tools = 20) was statistically significant ( p ≤ 0.001), indicative of a large ( d = 0.80, CI 0.71–0.90) reduction in symptom severity. Heterogeneity was large and statistically significant [I 2 = 97.51%, Q(df = 68) = 1328.96, p ≤ 0.001]. The funnel plot shows limited evidence of asymmetry. The funnel rank correlation test was not significant ( \(\tau\) = 0.009, p = 0.888). In contrast, the funnel regression test was statistically significant (Z = 2.533, p = 0.011). The fail-safe N was 121,899.
The random-effects meta-analysis for other outcomes ( k = 184, N = 126,734, measurement tools = 40) was statistically significant ( p ≤ 0.001), indicative of a large ( d = 1.01, CI 0.93–1.09) reduction in severity of indices of distress. Heterogeneity was large and statistically significant [I 2 = 99.06%, Q(df = 157) = 15,330.32, p ≤ 0.001]. The funnel plot shows a degree of asymmetry with clustering to the right of the mid-line. The funnel rank correlation test was statistically significant ( \(\tau\) = 0.208, p ≤ 0.001). In contrast, the funnel regression test was not significant (Z = 3.697, p ≤ 0.001). The fail-safe N was 1,695,607.
Moderator Analyses
Multivariable meta-regressions were conducted for each of the three outcome domains (Tables 3 , 4 , 5 ). After controlling for other moderators, the depression meta-regression found a significant effect for geographical region, therapist experience and type of analysis. UK samples had larger effect sizes compared to samples from Asia; effects sizes in samples treated by qualified staff members were larger than those observed in samples exclusively consisting of trainees; and samples excluding patients lost to follow-up (i.e., completer analyses) had larger effect sizes compared to intention-to-treat analyses. For anxiety outcomes, UK studies had larger effect sizes than studies from mainland Europe; mild severity samples had larger effect sizes than samples of patients with moderate or severe symptoms; and cognitive-behavioural interventions had larger effect sizes than counselling interventions. Finally, for other outcomes, the only significant moderator indicated that cognitive-behavioural interventions had larger effect sizes than psychodynamic interventions and unspecified (i.e., other) interventions.
Benchmarking Data
Pooled effect-sizes for low, average and high performing services are shown in Table 6 , organized according to setting [outpatient services, inpatient services, university counselling services (i.e., student population) and university psychotherapy clinics (non-student population)]. Although the effect size estimates for each benchmark vary across settings, confidence intervals consistently overlapped, indicating similar levels of symptom-changes across the performance strata (low, average, high). The exception to this is the low performance benchmark for anxiety measures which were significantly larger in university psychotherapy clinics ( d = 0.51) and significantly smaller in inpatient services ( d = 0.13) by comparison to outpatient services ( d = 0.37).
This review provides a comprehensive quantitative review of the effectiveness of psychological treatments delivered in routine care settings. Overall, 252 studies (samples k = 298) were identified, of which 223 (88.5%, k = 263) were included in the meta-analysis. Consistent with prior psychotherapy effectiveness reviews, we found large uncontrolled (pre–post treatment) effect sizes ( d = 0.80–1.01) across multiple outcome domains (depression, anxiety, and general psychological distress).
Consistent with previous meta-analyses of PBE (e.g., Cahill et al., 2010 ; Hunsley & Lee, 2007 ; Wakefield et al., 2021 ), we observed wide variability in effect sizes across studies and large (> 90%) indices of heterogeneity across outcome domains. The large number of samples included in this review enabled us to carry out adequately-powered moderator analyses to better understand potential sources of heterogeneity. For depression outcomes, smaller effect sizes were found for samples in Asia (compared to the UK), and in treatments delivered by trainees (i.e., compared to qualified professionals). For anxiety outcomes, smaller effect sizes were found for treatments delivered in mainland Europe (compared to the UK), services treating patients with moderate or high levels of severity (compared to mild severity), and counselling interventions (compared to cognitive-behavioural interventions). For other outcomes, only therapy modality was significant. Psychodynamic and unspecified interventions produced smaller effect-sizes (compared to cognitive-behavioural interventions). To some extent, these results are consistent with and support clinical guidelines that recommend cognitive-behavioural therapy as a first-line intervention, prior to considering other treatment modalities (National Institute for Health & care Excellence, 2011 ). However, caution is advised when interpreting these between-therapy comparisons using uncontrolled data from observational studies, as they could be explained by other unmeasured factors such as relevant case-mix differences between patients (e.g., socioeconomic status, personality, comorbid physical illnesses, etc.). Studies that control for case-mix variables using individual patient data find that there are no significant differences in treatment effects when comparing different treatment modalities (e.g., Pybis et al., 2017 ). Furthermore, as found in a previous meta-analysis (Wakefield et al., 2021 ), completers analyses tended to produce inflated (biased) effect sizes by comparison to intention-to-treat (more conservative and stringent) analyses.
The finding of large clinical improvements during psychotherapy and across outcomes was consistent with prior meta-analyses of psychotherapy effectiveness for depression outcomes (Hans & Hiller, 2013 ; Wakefield et al., 2021 ), anxiety outcomes (Stewart & Chambless, 2009 ; Wakefield et al., 2021 ), and other indices of psychological distress and functioning (Cahill et al., 2010 ). Pooled uncontrolled effect-sizes were smaller than that reported by Cahill et al. ( 2010 ) ( d = 1.29), although this may reflect differences in the focus of the reviews (e.g., Cahill et al., 2010 included group treatments) or the changing distribution of geographical representation (i.e., more studies from non-UK/North American countries). Large clinical improvements are also consistent with many meta-analyses of psychotherapy controlled trials (e.g., Cuijpers et al., 2008 , 2014a ; Mayo-Wilson et al., 2014 ; Olatunji et al., 2014 ).
It is possible that there are continental differences in models of training, service structures, therapy provision and emphasis on evidence-based practice which underlie the observed differences in pooled effect-sizes between continents. This is consistent with UK and US clinical guidance recommending delivery of empirically supported treatments (APA, 2006 ; National Institute for Health and Care Excellence, 2011 ). It is possible that the service policy context in the UK places greater emphasis on the delivery of treatment with high fidelity to empirically supported treatment protocols, and this may explain the relatively larger effect sizes in this geographical location, since high integrity is associated with better treatment outcomes and especially for anxiety treatment outcomes (Power et al., 2022 ). Despite these differences, all continents demonstrated positive change for all outcomes ( d = 0.59–1.10) supporting the universality hypothesis (i.e., that psychotherapy is assumed to work across cultures; Flückiger et al., 2018 ).
Consistent with several prior meta-analytic reviews (e.g., Cuijpers et al., 2014b ; Driessen et al., 2010 ; Furukawa et al., 2017 ), symptom severity did not predict effectiveness of treatment for depression. For anxiety outcomes, services categorized as treating mild conditions consistently had larger effect sizes. It is possible that classifying by type of service provided an imprecise proxy for sample severity and therefore future research should explore severity as a continuous variable in routine settings.
Limitations
The most notable critique of this review is that it is based exclusively on evidence from observational studies. We are unable to rule out alternative explanations for observed effect sizes [placebo effects, spontaneous remission (Posternak & Miller, 2001 ; Whiteford et al., 2012 )] and subsequently the observed effect sizes in this review cannot be directly compared to efficacy trials. Nevertheless, pooled effect sizes from observational studies serve as a valuable data source for benchmarking of routine care and quality improvement initiatives (e.g., Clark et al., 2018 ; Delgadillo et al., 2014 ; Gyani et al., 2013 ).
A key design limitation concerns statistical dependency. Efforts to avoid statistical dependency included: (i) taking one sample measure per domain, (ii) aggregating multiple unique study samples within a single domain, and (iii) extracting one measurement tool per study, per construct (i.e., preference system). These approaches have well-documented limitations (Borenstein et al., 2021 ; Hoyt & Del Re, 2018 ; Van den Noortgate et al., 2013 ). A preferable approach would have been to model dependency using a multi-level analysis (Van den Noortgate et al., 2013 , 2015 ) or through robust variance estimation and should be considered for future replications. Use of robust-variance estimation would avoid the need to assign outcomes to a restrictive number of outcome domains. This would also circumvent the need to adopt a highly heterogeneous “other” outcome domain, which for the current review included both diagnosis specific and global distress-based measures.
An additional limitation concerns the inherent limitations of the risk-of-bias assessment tool which was selected for this study a priori. It could be argued that this tool primarily indexes manuscript reporting detail and not necessarily risk of bias. Future reviews of effectiveness could consider assessing methodological rigour using other available rating tools (e.g., see Munder & Barth, 2018 ).
Due to resource constraints and the large number of included studies, the systematic search, data extraction and risk-of-bias ratings were not performed completely in duplicate. For the subsample of full texts screened by two coders there was a strong, but imperfect, agreement/reliability (80%, \(\kappa\) = 0.65). Similarly, not extracting data or assessing RoB in duplicate is problematic due to risk of imprecise estimates of treatment effect and RoB (Armijo-Olivo et al., 2014 ). An additional limitation surrounds coding decisions for moderator variables. Therapy modality was coded from manuscript self-definition. The degree to which treatments truly resembled treatment code (or treatment intended) is not clear. It was also apparent during extraction that very few practice-based studies report fidelity/adherence checks. As this becomes more routinely reported opportunities for modelling differences based on adherence/competence/integrity will become available. The use of categorical moderator levels to differentiate samples at the study level may also have provided imprecise proxies for moderator levels. For example, patient severity would preferably be modelled through meta-regression at the patient level to account for the heterogeneity within samples as it has been shown that university counselling center samples have numerous highly distressed individuals (Xiao et al., 2017 ). Future studies investigating these moderator variables at the patient level (e.g., through individual participant data meta-analysis) would help to shed light on this.
The search strategy is unlikely to have identified every available study. Search terms were based on prior reviews and omitted several terms that were found to produce an unmanageable number of records (e.g., “effectiveness”, “evaluation”). Despite this, the current reviews gives an adequate range and depth of effectiveness research with which to make tentative interpretations regarding the field of psychotherapy effectiveness research. A final caveat is the decision to focus exclusively on self-report measures of effectiveness. Meta-analytic evidence has demonstrated significant differences between self-report and clinician rated measures of clinical improvement (Cuijpers et al., 2010 ). Future research is therefore needed to see if the pooled effect-sizes from this study are consistent with clinician-rated measures of effectiveness in routine settings.
Conclusions
This review provides support for the effectiveness of psychological therapy as delivered in routine settings across a range of outcomes. Overall, the effects of psychotherapy appear to generalize well to diverse clinical settings, contexts, and populations. Nevertheless, it is evident that treatment effects vary considerably across services, and this review provides performance benchmarks to support routine service evaluation and practice development initiatives.
Data Availability
Data for the systematic review and related code will all be made publicly available through the lead author’s GitHub account following publication.
Standards of Reporting
This review followed the PRISMA and MAP-24 reporting guidelines for conducting systematic review/meta-analysis.
Protocol available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=175235 .
The authors recognise that use of the term effectiveness may be somewhat misleading. The pre–post (uncontrolled) methodology which forms the body of evidence in this review is unable to disentangle treatments effects from other potential causes of change (e.g., regression to the mean, placebo). Observed change in symptoms may therefore not exclusively represent treatment effectiveness. We have opted to retain use of this term within the current review because it has consistently and frequently been used as such in the extant literature (e.g., Lambert, 2013 ; Nordmo et al., 2020 ).
University clinics refers to university managed clinics treating communities beyond the student population. University counselling centres that are more specifically targeted at the student population are included within the mild category.
Addis, M. E., & Krasnow, A. D. (2000). A national survey of practicing psychologists’ attitudes toward psychotherapy treatment manuals. Journal of Consulting and Clinical Psychology, 68 (2), 331–339. https://doi.org/10.1037/0022-006X.68.2.331
Article CAS Google Scholar
APA. (2006). Evidence-based practice in psychology. Presidential Task Force on Evidence-Based Practice. American Psychologist, 61 , 271–285.
Article Google Scholar
Armijo-Olivo, S., Ospina, M., da Costa, B. R., Egger, M., Saltaji, H., Fuentes, J., Ha, C., & Cummings, G. G. (2014). Poor reliability between Cochrane reviewers and blinded external reviewers when applying the Cochrane risk of bias tool in physical therapy trials. PLoS ONE, 9 (5), e96920. https://doi.org/10.1371/journal.pone.0096920
Balk, E. M., Earley, A., Patel, K., Trikalinos, T. A., & Dahabreh, I. J. (2012). Empirical assessment of within-arm correlation imputation in trials of continuous outcomes. Methods Research Reports, 12 (13), EHC141-EF.
Google Scholar
Barkham, M., Margison, F., Leach, C., Lucock, M., Mellor-Clark, J., Evans, C., Benson, L., Connell, J., Audin, K., & McGrath, G. (2001). Service profiling and outcomes benchmarking using the CORE-OM: Toward practice-based evidence in the psychological therapies. Journal of Consulting and Clinical Psychology, 69 (2), 184–196. https://doi.org/10.1037/0022-006X.69.2.184
Barkham, M., Stiles, W. B., Lambert, M. J., & Mellor-Clark, J. (2010). Building a rigorous and relevant knowledge base for the psychological therapies. In M. Barkham, G. E. Hardy, & J. Mellor-Clark (Eds.), Developing and delivering practice-based evidence (pp. 21–61). Wiley. https://doi.org/10.1002/9780470687994.ch2
Chapter Google Scholar
Becker, B. J. (1988). Synthesizing standardized mean-change measures. British Journal of Mathematical & Statistical Psychology, 41 (2), 257–278. https://doi.org/10.1111/j.2044-8317.1988.tb00901
Begg, C. B., & Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50 (4), 1088–1101. https://doi.org/10.2307/2533446
Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2021). Introduction to meta-analysis . Wiley.
Book Google Scholar
Cahill, J., Barkham, M., & Stiles, W. (2010). Systematic review of practice-based research on psychological therapies in routine clinic settings. The British Journal of Clinical Psychology, 49 (4), 421–453. https://doi.org/10.1348/014466509X470789
Card, N. A. (2015). Applied meta-analysis for social science research . Guilford Publications.
Castonguay, L. G., Barkham, M., Jeong Youn, S., & Page, A. C. (2021). Practice-based evidence findings from routine clinical settings. In M. Barkham, W. Lutz, & L. G. Castonguay (Eds.), Bergin and Garfield’s handbook of psychotherapy and behavior change (7th ed., pp. 191–222). Wiley.
Castonguay, L. G., Barkham, M., Lutz, W., & McAleavey, A. A. (2013). Practice-orienIrach: Approaches and applications. In M. J. Lambert (Ed.), Bergin and Garfield’s handbook of psychotherapy and behavior change (6th ed., pp. 85–133). Wiley.
Chambless, D. L., & Hollon, S. D. (1998). Defining empirically supported therapies. Journal of Consulting and Clinical Psychology, 66 (1), 7–18. https://doi.org/10.1037//0022-006x.66.1.7
Chambless, D. L., & Ollendick, T. H. (2001). Empirically supported psychological interventions: Controversies and evidence. Empirically Supported Psychological Interventions: Controversies and Evidence, 52 (1), 685–716. https://doi.org/10.1146/annurev.psych.52.1.685
Clark, D. M., Canvin, L., Green, J., Layard, R., Pilling, S., & Janecka, M. (2018). Transparency about the outcomes of mental health services (IAPT approach): An analysis of public data. The Lancet, 391 (10121), 679–686. https://doi.org/10.1016/S0140-6736(17)32133-5
Cochran, W. G. (1954). The combination of estimates from different experiments. Biometrics, 10 (1), 101–129. https://doi.org/10.2307/3001666
Cohen, J. (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20 (1), 37–46. https://doi.org/10.1177/001316446002000104
Connell, J., Barkham, M., & Mellor-Clark, J. (2007). CORE-OM mental health norms of students attending university counselling services benchmarked against an age-matched primary care sample. British Journal of Guidance & Counselling, 35 (1), 41–57. https://doi.org/10.1080/03069880601106781
Cooper, H. M. (1998). Synthesizing research: A guide for literature reviews . SAGE.
Cuijpers, P. (2016). Meta-analyses in mental health research: A practical guide . University of Amsterdam.
Cuijpers, P., Li, J., Hofmann, S. G., & Andersson, G. (2010). Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: A meta-analysis. Clinical Psychology Review, 30 (6), 768–778. https://doi.org/10.1016/j.cpr.2010.06.001
Cuijpers, P., Sijbrandij, M., Koole, S., Huibers, M., Berking, M., & Andersson, G. (2014a). Psychological treatment of generalized anxiety disorder: A meta-analysis. Clinical Psychology Review, 34 (2), 130–140. https://doi.org/10.1016/j.cpr.2014.01.002
Cuijpers, P., Turner, E. H., Mohr, D. C., Hofmann, S. G., Andersson, G., Berking, M., & Coyne, J. (2014b). Comparison of psychotherapies for adult depression to pill placebo control groups: A meta-analysis. Psychological Medicine, 44 (4), 685–695. https://doi.org/10.1017/S0033291713000457
Cuijpers, P., van Straten, A., Andersson, G., & van Oppen, P. (2008). Psychotherapy for depression in adults: A meta-analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology, 76 (6), 909–922. https://doi.org/10.1037/a0013075
Delgadillo, J., McMillan, D., Leach, C., Lucock, M., Gilbody, S., & Wood, N. (2014). Benchmarking routine psychological services: A discussion of challenges and methods. Behavioural and Cognitive Psychotherapy, 42 (1), 16–30. https://doi.org/10.1017/S135246581200080X
Driessen, E., Cuijpers, P., Hollon, S. D., & Dekker, J. J. M. (2010). Does pretreatment severity moderate the efficacy of psychological treatment of adult outpatient depression? A meta-analysis. Journal of Consulting and Clinical Psychology, 78 (5), 668–680. https://doi.org/10.1037/a0020570
Egger, M., Smith, G. D., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315 (7109), 629–634. https://doi.org/10.1136/bmj.315.7109.629
Flückiger, C., Del Re, A. C., Barth, J., Hoyt, W. T., Levitt, H., Munder, T., Spielmans, G. I., Swift, J. K., Vîslă, A., & Wampold, B. E. (2018). Considerations of how to conduct meta-analyses in psychological interventions. Psychotherapy Research, 28 (3), 329–332. https://doi.org/10.1080/10503307.2018.1430390
Freedland, K. E., Mohr, D. C., Davidson, K. W., & Schwartz, J. E. (2011). Usual and unusual care: Existing practice control groups in randomized controlled trials of behavioral interventions. Psychosomatic Medicine, 73 (4), 323–335. https://doi.org/10.1097/PSY.0b013e318218e1fb
Furukawa, T. A., Weitz, E. S., Tanaka, S., Hollon, S. D., Hofmann, S. G., Andersson, G., Twisk, J., DeRubeis, R. J., Dimidjian, S., Hegerl, U., Mergl, R., Jarrett, R. B., Vittengl, J. R., Watanabe, N., & Cuijpers, P. (2017). Initial severity of depression and efficacy of cognitive-behavioural therapy: Individual-participant data meta-analysis of pill-placebo-controlled trials. British Journal of Psychiatry, 210 (3), 190–196. https://doi.org/10.1192/bjp.bp.116.187773
Gleser, L. J., & Olkin, I. (2009). Stochastically dependent effect sizes. In H. Cooper, L. V. Hedges, & J. C. Valentine (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 357–376). Russell Sage.
Gyani, A., Shafran, R., Layard, R., & Clark, D. M. (2013). Enhancing recovery rates: Lessons from year one of IAPT. Behaviour Research and Therapy, 51 (9), 597–606. https://doi.org/10.1016/j.brat.2013.06.004
Hans, E., & Hiller, W. (2013). Effectiveness of and dropout from outpatient cognitive behavioral therapy for adult unipolar depression: A meta-analysis of nonrandomized effectiveness studies. Journal of Consulting and Clinical Psychology, 81 (1), 75–88. https://doi.org/10.1037/a0031080
Harrer, M., Cuijpers, P., Furukawa, T. A., & Ebert, D. D. (2019). Dmetar: Companion R package for the guide ‘doing meta-analysis in R’. [R Package].
Higgins, J., & Thompson, S. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21 (11), 1539–1558. https://doi.org/10.1002/sim.1186
Hoyt, W. T., & Del Re, A. C. (2018). Effect size calculation in meta-analyses of psychotherapy outcome research. Psychotherapy Research, 28 (3), 379–388. https://doi.org/10.1080/10503307.2017.1405171
Hunsley, J., & Lee, C. M. (2007). Research-informed benchmarks for psychological treatments: Efficacy studies, effectiveness studies, and beyond. Professional Psychology: Research and Practice, 38 (1), 21–33. https://doi.org/10.1037/0735-7028.38.1.21
Jüni, P., Altman, D. G., & Egger, M. (2001). Assessing the quality of controlled clinical trials. BMJ, 323 (7303), 42–46.
Lambert, M. J. (2013). The efficacy and effectiveness of psychotherapy. In M. J. Lambert & A. E. Bergin (Eds.), Bergin and Garfield’s handbook of psychotherapy and behavior change (6th ed., pp. 169–218). Wiley.
Lemma, A., Roth, A. D., & Pilling, S. (2008). The competences required to deliver effective psychoanalytic/psychodynamic therapy . Research Department of Clinical, Educational and Health Psychology, University College London.
Lewis, C., Roberts, N. P., Andrew, M., Starling, E., & Bisson, J. I. (2020). Psychological therapies for post-traumatic stress disorder in adults: Systematic review and meta-analysis. European Journal of Psychotraumatology, 11 (1), 1729633. https://doi.org/10.1080/20008198.2020.1729633
Linardon, J., Wade, T. D., de la Piedad Garcia, X., & Brennan, L. (2017). The efficacy of cognitive-behavioral therapy for eating disorders: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology, 85 (11), 1080–1094. https://doi.org/10.1037/ccp0000245
Lutz, W., Schiefele, A.-K., Wucherpfennig, F., Rubel, J., & Stulz, N. (2016). Clinical effectiveness of cognitive behavioral therapy for depression in routine care: A propensity score based comparison between randomized controlled trials and clinical practice. Journal of Affective Disorders, 189 (1), 150–158. https://doi.org/10.1016/j.jad.2015.08.072
Margison, F. R., Barkham, M., Evans, C., McGrath, G., Clark, J. M., Audin, K., & Connell, J. (2000). Measurement and psychotherapy: Evidence-based practice and practice-based evidence. British Journal of Psychiatry, 177 (2), 123–130. https://doi.org/10.1192/bjp.177.2.123
Mayo-Wilson, E., Dias, S., Mavranezouli, I., Kew, K., Clark, D. M., Ades, A. E., & Pilling, S. (2014). Psychological and pharmacological interventions for social anxiety disorder in adults: A systematic review and network meta-analysis. The Lancet Psychiatry, 1 (5), 368–376. https://doi.org/10.1016/S2215-0366(14)70329-3
McAleavey, A. A., Youn, S. J., Xiao, H., Castonguay, L. G., Hayes, J. A., & Locke, B. D. (2019). Effectiveness of routine psychotherapy: Methods matters. Psychotherapy Research, 29 , 139–156.
Minami, T., Serlin, R. C., Wampold, B. E., Kircher, J. C., & Brown, G. S. (2008). Using clinical trials to benchmark effects produced in clinical practice. Quality and Quantity, 42 (4), 513–525. https://doi.org/10.1007/s11135-006-9057-z
Morris, S. B. (2008). Estimating effect sizes from pretest–posttest-control group designs. Organizational Research Methods, 11 (2), 364–386. https://doi.org/10.1177/1094428106291059
Munder, T., & Barth, J. (2018). Cochrane’s risk of bias tool in the context of psychotherapy outcome research. Psychotherapy Research, 28 (3), 347–355. https://doi.org/10.1080/10503307.2017.1411628
Munn, Z., Barker, T. H., Moola, S., Tufanaru, C., Stern, C., McArthur, A., Stephenson, M., & Aromataris, E. (2020). Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evidence Synthesis, 18 (10), 2127–2133. https://doi.org/10.11124/JBISRIR-D-19-00099
National Institute for Health and Care Excellence. (2011). Common mental health problems: Identification and pathways to care (CG90). https://www.nice.org.uk/guidance/cg123/chapter/1-guidance
Nordmo, M., Sønderland, N. M., Havik, O. E., Eilertsen, D.-E., Monsen, J. T., & Solbakken, O. A. (2020). Effectiveness of open-ended psychotherapy under clinically representative conditions. Frontiers in Psychiatry, 11 , 384. https://doi.org/10.3389/fpsyt.2020.00384
Olatunji, B. O., Kauffman, B. Y., Meltzer, S., Davis, M. L., Smits, J. A. J., & Powers, M. B. (2014). Cognitive-behavioral therapy for hypochondriasis/health anxiety: A meta-analysis of treatment outcome and moderators. Behaviour Research and Therapy, 58 , 65–74. https://doi.org/10.1016/j.brat.2014.05.002
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., …, Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLOS Medicine, 18 (3), e1003583. https://doi.org/10.1371/journal.pmed.1003583
Persons, J. B., Bostrom, A., & Bertagnolli, A. (1999). Results of randomized controlled trials of cognitive therapy for depression generalize to private practice. Cognitive Therapy and Research, 23 (5), 535–548. https://doi.org/10.1023/A:1018724505659
Posternak, M. A., & Miller, I. (2001). Untreated short-term course of major depression: A meta-analysis of outcomes from studies using wait-list control groups. Journal of Affective Disorders, 66 (2–3), 139–146. https://doi.org/10.1016/s0165-0327(00)00304-9
Power, N., Noble, L., Simmonds-Buckley, M., Kellett, S., Stockton, C., Firth, N., & Delgadillo, J. (2022). Associations between treatment adherence-competence-integrity (ACI) and adult psychotherapy outcomes: A systematic review and meta-analysis. Journal of Consulting and Clinical Psychology, 90 (5), 427–445. https://doi.org/10.1037/ccp0000736
Pybis, J., Saxon, D., Hill, A., & Barkham, M. (2017). The comparative effectiveness and efficiency of cognitive behaviour therapy and generic counselling in the treatment of depression: Evidence from the 2nd UK National Audit of psychological therapies. BMC Psychiatry, 17 (1), 1–13. https://doi.org/10.1186/s12888-017-1370-7
R Core Team. (2020). R: A language and environment for statistical computing . R Foundation for Statistical Computing. https://www.R-project.org/
Rosa-Alcázar, A. I., Sánchez-Meca, J., Gómez-Conesa, A., & Marín-Martínez, F. (2008). Psychological treatment of obsessivecompulsive disorder: A meta-analysis. Clinical Psychology Review, 28 (8), 1310–1325. https://doi.org/10.1016/j.cpr.2008.07.001
Rosenthal, R. (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86 (3), 638–641. https://doi.org/10.1037/0033-2909.86.3.638
Roth, A., Hill, A., & Pilling, S. (2009). The competences required to deliver effective humanistic psychological therapies . University College London.
Roth, A., & Pilling, S. (2008). Using an evidence-based methodology to identify the competences required to deliver effective cognitive and behavioural therapy for depression and anxiety disorders. Behavioural and Cognitive Psychotherapy, 36 (2), 129–147. https://doi.org/10.1017/S1352465808004141
Sánchez-Meca, J., Rosa-Alcázar, A. I., Marín-Martínez, F., & Gómez-Conesa, A. (2010). Psychological treatment of panic disorder with or without agoraphobia: A meta-analysis. Clinical Psychology Review, 30 (1), 37–50. https://doi.org/10.1016/j.cpr.2009.08.011
Schwarzer, G. (2020). Meta: General package for meta-analysis . https://CRAN.R-project.org/package=meta
Shadish, W. R., Matt, G. E., Navarro, A. M., Siegle, G., Crits-Christoph, P., Hazelrigg, M. D., Jorm, A. F., Lyons, L. C., Nietzel, M. T., Robinson, L., Prout, H. T., Smith, M. L., Svartberg, M., & Weiss, B. (1997). Evidence that therapy works in clincally representative conditions. Journal of Consulting and Clinical Psychology, 65 (3), 355–365. https://doi.org/10.1037/0022-006X.65.3.355
Shadish, W. R., Navarro, A. M., Matt, G. E., & Phillips, G. (2000). The effects of psychological therapies under clinically representative conditions: A meta-analysis. Psychological Bulletin, 126 (4), 512–529. https://doi.org/10.1037/0033-2909.126.4.512
Spielmans, G. I., & Flückiger, C. (2018). Moderators in psychotherapy meta-analysis. Psychotherapy Research, 28 (3), 333–346. https://doi.org/10.1080/10503307.2017.1422214
Stewart, R. E., & Chambless, D. L. (2009). Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: A meta-analysis of effectiveness studies. Journal of Consulting and Clinical Psychology, 77 (4), 595–606. https://doi.org/10.1037/a0016032
Van den Noortgate, W., López-López, J. A., Marín-Martínez, F., & Sánchez-Meca, J. (2013). Three-level meta-analysis of dependent effect sizes. Behavior Research Methods, 45 (2), 576–594. https://doi.org/10.3758/s13428-012-0261-6
Van den Noortgate, W., López-López, J. A., Marín-Martínez, F., & Sánchez-Meca, J. (2015). Meta-analysis of multiple outcomes: A multilevel approach. Behavior Research Methods, 47 (4), 1274–1294. https://doi.org/10.3758/s13428-014-0527-2
van der Lem, R., de Wever, W. W., van der Wee, N. J., van Veen, T., Cuijpers, P., & Zitman, F. G. (2012). The generalizability of psychotherapy efficacy trials in major depressive disorder: An analysis of the influence of patient selection in efficacy trials on symptom outcome in daily practice. BMC Psychiatry, 12 (1), 192. https://doi.org/10.1186/1471-244X-12-192
Viechtbauer, W. (2020). Metafor: Meta-analysis package for r . https://CRAN.R-project.org/package=metafor
Wakefield, S., Kellett, S., Simmonds-Buckley, M., Stockton, D., Bradbury, A., & Delgadillo, J. (2021). Improving Access to Psychological Therapies (IAPT) in the United Kingdom: A systematic review and meta-analysis of 10-years of practice-based evidence. British Journal of Clinical Psychology, 60 (1), 1–37. https://doi.org/10.1111/bjc.12259
Weisz, J. R., Weiss, B., Han, S. S., Granger, D. A., & Morton, T. (1995). Effects of psychotherapy with children and adolescents revisited: A meta-analysis of treatment outcome studies. Psychological Bulletin, 117 (3), 450–468. https://doi.org/10.1037/0033-2909.117.3.450
Whiteford, H., Harris, M., Mckeon, G., Baxter, A., Pennell, C., Barendregt, J., & Wang, J. (2012). Estimating remission from untreated major depression: A systematic review and meta-analysis. Psychological Medicine, 43 , 1–17. https://doi.org/10.1017/S0033291712001717
Wolitzky-Taylor, K. B., Horowitz, J. D., Powers, M. B., & Telch, M. J. (2008). Psychological approaches in the treatment of specific phobias: A meta-analysis. Clinical Psychology Review, 28 (6), 1021–1037. https://doi.org/10.1016/j.cpr.2008.02.007
Xiao, H., Carney, D. M., Youn, S. J., Janis, R. A., Castonguay, L. G., Hayes, J. A., & Locke, B. D. (2017). Are we in crisis? National mental health and treatment trends in college counseling centers. Psychological Services, 14 (4), 407–415. https://doi.org/10.1037/ser0000130
Zimmerman, M., Mattia, J. I., & Posternak, M. A. (2002). Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? American Journal of Psychiatry, 159 (3), 469–473. https://doi.org/10.1176/appi.ajp.159.3.469
Download references
Acknowledgements
We would like to thank Dr Gregg Rawlings for his work in second rating at the screening stage for titles and abstracts.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Author information
Authors and affiliations.
University of Sheffield, Sheffield, UK
Chris Gaskell, Melanie Simmonds-Buckley, Stephen Kellett, C. Stockton, Erin Somerville, Emily Rogerson & Jaime Delgadillo
Sheffield Health and Social Care NHS Foundation Trust, Sheffield, UK
Stephen Kellett
You can also search for this author in PubMed Google Scholar
Contributions
The first author (CG) for the study was responsible for systematic search, analysis, and led on the writing of the manuscript. The second author (MS-B) provided academic supervision, guidance on methodology/analysis, and commented on manuscripts. The third author (SK) provided overall project supervision, guidance on methodology/analysis, and commented on manuscripts. The fourth author (CS) conducted second ratings at the stages of full-text screening and data extraction. The fifth (ES) and sixth (ER) authors conducted second ratings for the risk of bias assessments. The seventh author (JD) provided methodological guidance and contributed to the writing of the manuscript.
Corresponding author
Correspondence to Chris Gaskell .
Ethics declarations
Competing interests.
The authors have no competing interests to declare that are relevant to the content of this article.
Consent to Participate
This systematic review did not involve participant recruitment.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 324 kb)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Gaskell, C., Simmonds-Buckley, M., Kellett, S. et al. The Effectiveness of Psychological Interventions Delivered in Routine Practice: Systematic Review and Meta-analysis. Adm Policy Ment Health 50 , 43–57 (2023). https://doi.org/10.1007/s10488-022-01225-y
Download citation
Accepted : 14 September 2022
Published : 06 October 2022
Issue Date : January 2023
DOI : https://doi.org/10.1007/s10488-022-01225-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Psychotherapy
- Effectiveness
- Naturalistic
- Routine outcomes
- Meta-analysis
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
Studies were excluded if they: used (e) family/group treatments, (f) were not available in English; (g) did not employ a self-report measure of treatment effectiveness2; (h) did not provide sufficient data to calculate pre–post treatment effect sizes; or (i) employed randomization procedures or control groups. A more detailed table of ...
A meta-analysis on the effectiveness of anger treatments for specific anger problems (Del Vecchio & O’Leary, 2004) included only studies in which subjects met clinically significant levels of anger on standardized anger measurements prior to treatment. This meta-analysis examined the effects of CBT, cognitive therapy, relaxation, and ‘other ...
Jan 11, 2013 · Background This systematic review describes a comparison between several standard treatments for major depressive disorder (MDD) in adult outpatients, with a focus on interpersonal psychotherapy (IPT). Methods Systematic searches of PubMed and PsycINFO studies between January 1970 and August 2012 were performed to identify (C-)RCTs, in which MDD was a primary diagnosis in adult outpatients ...
Mental disorders represent a worldwide public health concern1, 2.Psychotherapies and pharmacotherapies are recommended as first line treatments3, 4.However, evidence has recently emerged suggesting that the efficacy of both types of treatment may have been overestimated, due to several shortcomings of clinical trials, such as publication bias, researcher allegiance, or use of weak comparison ...
WHEREAS: the research continues to support that psychotherapy, both group and individuals models of clinical interventions, is effective treatment for individuals with disabilities. The studies also indicate that psychotherapy is effective for a variety of disability conditions including cognitive, intellectual, physical, visual, auditory, and ...
Orientation psychoanalytical psychotherapies effective on all measurements at 6 and 18 months. IT (psychodynamic) effective on diagnostic criteria, maintained from 1 to 5 years; individual and group therapies effective. Antisocial personality: 1 controlled trial: Brief psychodynamic therapy beneficial to patients presenting with depression.
Coping with depression: a course, comparison of group or class therapy and individual therapy (2 studies): Meta-analysis showed individual treatment was significantly more effective at reducing BDI scores than was the class method (n=104; WMD 3.0, 95% CI 1.0, 5.0), however the effect did not persist at follow up at 1 and 6 months.
Apr 30, 2018 · Five years later Levitt (1957) reviewed the research on child and adolescent psychotherapy and reached a verdict similar to that of Eysenck: “. . . the results of the present study fail to support the view that psychotherapy with ‘neurotic’ children is effective” (p. 195).
Oct 6, 2022 · This review presents a comprehensive evaluation of the effectiveness of routinely delivered psychological therapies across inpatient, outpatient and University-based clinics. This was a pre-registered systematic-review of studies meeting pre-specified inclusion criteria (CRD42020175235). Eligible studies were searched in three databases: MEDLINE, CINAHL and PsycInfo. Pre–post treatment ...
Research studies evaluating how effective individual therapies are at treating particular disorders have commonly reported: significant differences among therapies. In preparation for a study of the effectiveness of an antipsychotic drug, an assistant puts all drugs into capsules of the same color and codes them.