3 Awesome Science Experiments With Fire!


Introduction: 3 Awesome Science Experiments With Fire!
Please be careful when you will be performing these fire experiments at home or at school. All of these fire tricks can be extremely dangerous so again, please be careful. Always use safety glasses or face-shield, gloves, well-ventilated areas and adult supervision. Its good to have prepared fire extinguisher.
Subscribe if you like ;-)
3 Awesome Science Experiments with Fire!
https://www.youtube.com/watch?v=zlVNs-yHm04
Step 1: Fire Bubbles Experiment

Fill a kitchen plate with ordinary tap water. Add a little of dish soap to the water. Submerge the open end of the butane gas tube in the soapy water and press. Butane gas will create bubbles which you can catch by hand.
Before catching the bubbles and light them with lighter or match, make sure that every part of your hands and wrists are covered with water to protect them from a burn and don't forget to put a plate with bubbles a bit far from the place where you will make an experiment. I was using lighter refill with butane gas , you can use same or methane gas.
Step 2: Fire Hands Experiment

Hand Sanitizer contains water, ethyl alcohol which is highly flammable and can contain some perfume. Ethanol, also called alcohol, ethyl alcohol, and drinking alcohol, is a chemical compound, a simple alcohol with the chemical formula C2H5OH. It also has medical applications as an antiseptic and disinfectant.
Gels that contain ethanol produce a relatively cool flame with the blue color because of a high percentage of the water in the product.
But keep in mind, that the flame is still hot enough to burn you if you hold it too long and can ignite paper, fabrics, etc. Use care to perform this experiment in a safe location, away from flammable material. As we recommended before, it's a good idea to have a fire extinguisher or at least a glass of water.
I recommend using this Hand Sanitizer.
Step 3: Traveling Flame

This is simple and easy fire trick with a candle that will surprise anyone who sees it. Almost every candle is made out the wax. When you light a candle, heat from flame melts wax close to the wick and the melted wax flows up inside the wick by capillary action.
The wax becomes a hot gas by heat from the flame and its hydrocarbons (CnH2n+2) break down into carbon (C) and hydrogen (H). The vaporized wax is burned with oxygen (O) and is producing water vapor (H2O), carbon dioxide (CO2), light, and heat.
Smoke from a candle is unburned wax vapor and substance called “soot” which is a black material composed mostly of carbon. For a few seconds, the temperature of the smoke is high enough that it will burn with the touch of a flame. Because smoke is hot, It rises and you would like to light it, you should be few inches above the wick.
I recommend using long candle like this , it's easier to light it.

The Experiment Archive
Fire experiments
Special: Colored fire
Special: Fire bubbles
Orange candle

Special: Fire tornado

Special: Whoosh bottle

Special: Dust explosion

Special: Burning money

Special: Burning towel
Carbon dioxide extuingisher

Special: Screaming gummy bear

Tea bag rocket

Fireproof balloon

Levitating match

Traveling flame
Content of website.

Fire Science Experiment - Teach kids about an important science concept of fire
Posted by Admin / in Chemistry Experiments
Exploding fire science experiments are wonderful features at live science museum shows. These experiments "wow" the crowd and help to show something about science, but they are not safe unless the building is set up correctly. This fire science experiment is not exploding, but is great for teaching kids about the science of fire.
Materials Needed
- Clear glass jar
- Antacid tablets (must contain sodium bicarbonate)
- Disposable cup
- Table knife or fork
- Small piece of wax-based clay
FIRE SCIENCE EXPERIMENT STEPS
Step 1: Remove the label and completely dry the inside of a clear glass jar. A spaghetti sauce jar works well.
Step 2: *An adult must handle the antacid tablets or an adult must provide close supervision while the kids help with the antacid tablets.* Take an antacid tablet out the package and place it in the bottom of a dry cup. Using the table knife or fork, chop up the antacid tablet into smaller pieces.

Using the table knife or fork, break the antacid tablets into smaller pieces in the bottom of a dry cup.
Step 3: Place a ball of clay on the bottom of the candle. Now press the unlit candle and clay into the bottom of the jar, inside the jar. Using tongs or long needle-nose pliers helps to grip the candle to press it against the glass at the bottom of the jar.
Step 4: Pour the broken antacid tablet pieces around the unlit candle.

Antacid pieces in the bottom of the jar surrounding the candle
Step 5: *An adult must handle lighting the candle.* Light the candle by turning the jar upside down. The flame from the match rises which is why holding the jar upside down helps allow the candle to be lit inside the jar. Turn the jar over and set it on the counter.

Have an adult carefully light the candle
Step 6: Tip the jar slightly and carefully pour the water in the jar around the candle, without pouring it over the flame.

Quickly, but carefully pour the water around the lit candle, but do not extinguish the flame
Step 7: Observe the reaction taking place within the mixture of water and tablet pieces.

The sodium bicarbonate reacts with water resulting in CO 2 gas. The bubbles in the jar is the result of CO 2 gas being produced.
Step 8: Watch the candle for the next minute or two. The candle will start to crackle, then eventually burn out.

As C0 2 gas is produced from the chemical reaction, other gases inside the jar are pushed out.
SCIENCE LEARNED
The bubbles you saw as soon as the water was added to the antacid pieces was carbon dioxide gas being released. Antacid contains sodium bicarbonate (NaHCO 3 ), which is also known as baking soda. Sodium bicarbonate is a weak base. When antacid is combined with water it reacts quickly, resulting in the release of sodium, water and carbon dioxide. Using smashed up pieces of the antacid helps speed up the reaction.
The second interesting thing that happened in this experiment was what happened to the fire. The lit candle in this experiment was using oxygen to continue to burn. When the carbon dioxide is released it starts to mix with the oxygen-rich air in the jar. Carbon dioxide gas is heavier than oxygen but this is not why the flame is extinguished. As more and more carbon dioxide gas is released by the antacid reaction there just is not enough oxygen left in the jar for the fire to continue. At first, the flame may crackle, but then finally it will stop burning.
Did you know that there are carbon dioxide fire extinguishers? CO 2 (carbon dioxide) fire extinguishers work by moving the oxygen away from the location of the fire, extinguishing the flames. Now that we have seen the results of the fire experiment we know why these types of fire extinguishers work well.
- About the author
- Back to Experiment
Please select the social network you want to share this page with:
We like you too :)
Thanks for taking time to give us feedback!
- chemistry experiments
- science experiments for kids
- experiments with fire
- science experiments with gas
- carbon dioxide gas experiment

posted by Admin
- previous experiment
- next experiment

Egg in Vinegar Experiment
in Chemistry Experiments
Experiment with the chemical reaction between vinegar and an eggshell.
Copper Plating Experiment
Experiment with copper ions by adding copper plating to an iron nail.

Stringy Goo Experiment
Make slimy, stringy goo with a gross, but fun chemistry experiment for kids.

Antioxidant Experiment
Use food to show kids different materials that are used to preserve food with this antioxidant experiment.


Lemon Battery Experiment
in Energy & Electricity Experiments
Use either lemons or potatoes to generate electricity. This experiment is a great to teach kids about energy storage.


Fire Extinguisher Science Experiment for Kids
This post may contain affiliate links.

Today I want to show you two really simple fire extinguisher science experiments for kids. These experiments need just a few basic supplies. They are really engaging and interesting! You can use them to teach kids the properties of fire and ways to safely extinguish them.

This week I am working on content about community helpers . This science experiment is perfect when teaching a lesson about firefighters to kids. It also would be great for a fire safety lesson.
My kids loved these fire experiments. We did each of them four or five times so they could watch them again and again!
Watch Them Here:
Fire Extinguisher Experiment #1: Candle Under a Jar

This first experiment is so simple it doesn’t really feel like a big deal. However, kids will be surprised! All you need is a small tea light candle , some matches and a jar or glass larger than the candle.

Light the candle. Place the jar over the candle and see how long it takes for the candle to blow out.

The Science Behind it:
When you put a candle under a jar, there will be a limited amount of oxygen under that jar. Fire burns on oxygen and when it runs out, it will not burn any longer. The flame will go out.

Experiment #2: DIY Fire Extinguisher
There are several types of fire extinguishers, just as there are different types of fires. Each of them put out fires in different ways. Some of them smother the fire. Some of them One way a fire can be put out is with carbon dioxide. This type is usually used with electrical fires. They release carbon dioxide into the air diluting the oxygen and causing the fire to burn out.
This experiment uses carbon dioxide created from the chemical reaction between baking soda and vinegar to put out a fire.
How to Make the DIY Fire Extinguisher:

For this experiment, you will need some small tea light candles, baking soda, vinegar, matches, a funnel and a bottle or pitcher with a lid.

In your bottle, pour in 3 tablespoons of baking soda. Next, pour in about 1/2 cup of white vinegar. Quickly seal the top of your bottle to seal in the gas created from the reaction.
Light your candles.

Allow the bubbling from the reaction to stop. Once it is done bubbling, tip the bottle over and open it while facing the lit candles. It will release the carbon dioxide gas and burn the candles right out!

This experiment feels a bit like a magic trick!
The Science Behind it:
Just like the previous experiment, when there is decreased oxygen, the fire will go out. With this experiment, the carbon dioxide is released pushing away the oxygen and quickly putting out the flames on the candles.
See More Fun Science Experiments for Kids:
100+ Easy and FUN Science Project Ideas for Kids
See Also our Rising Tide Experiment with Fire !
Former school teacher turned homeschool mom of 4 kids. Loves creating awesome hands-on creative learning ideas to make learning engaging and memorable for all kids!
Similar Posts

How to Make Oobleck: Science with Dr. Seuss!

Melting Snowmen: Acetone and Styrofoam Slime Experiment

EASY Play Dough Circuits
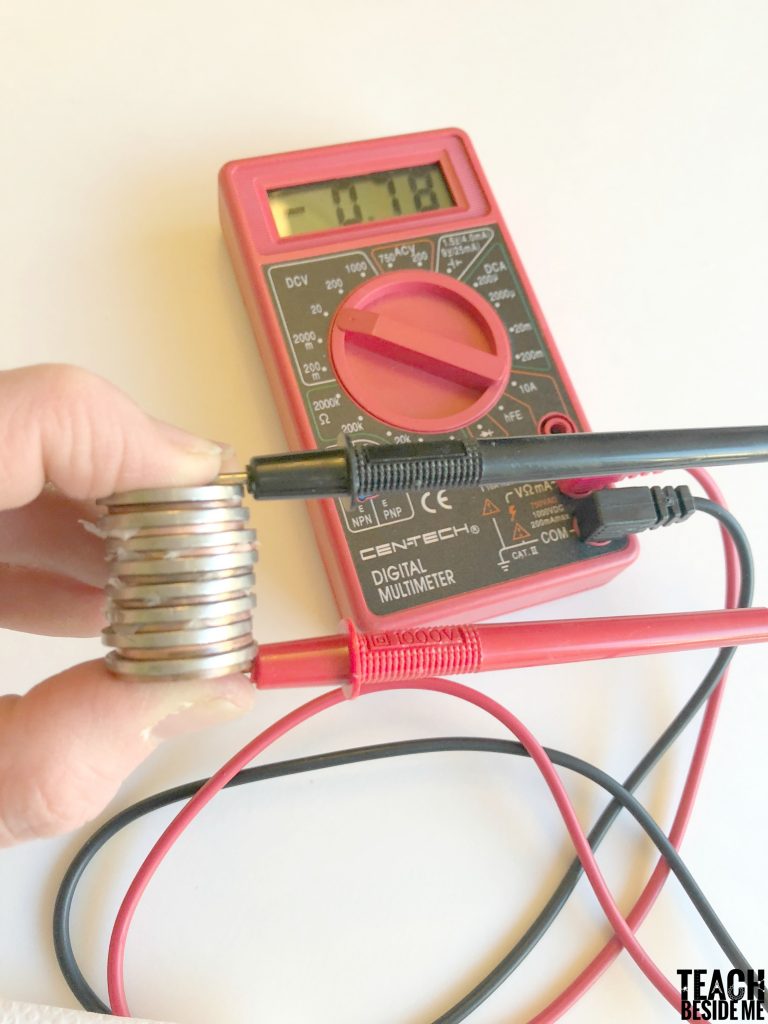
STEM for Kids: How to Make a Coin Battery

Edible Bubble Gum Slime

How to Make Slime ~ The Best and Easiest Recipe
I teach science and these are great experiments for light and science. Thank you!
So glad to hear! Thanks for your comment!
Leave a Reply Cancel reply
You must be logged in to post a comment.

Oxygen and Fire Experiment
Sharing is caring!

Oxygen is a vital component of the air in our atmosphere. We need to breathe it in to survive, as do most other organisms. It is also necessary for combustion reactions, such as fire. In this oxygen and fire experiment, we will test the relationship between oxygen and fire.
– large glass jar
– candle
– matches
– 2 cups of hydrogen peroxide
– baker’s yeast
– a bottle (1 liter soda bottle or wine bottle will work)
– a balloon
– a teaspoon
– safety glasses
Put on your safety glasses.
Fill your bottle with approximately 2 cups of hydrogen peroxide. Then, add about 1 teaspoon of yeast to the peroxide. Quickly open the balloon over the opening of the bottle making sure no air can escape.

Gently, shake the bottle back and forth to mix the yeast and peroxide. You should now see bubbles beginning to form. These are oxygen bubbles created by the decomposition of the hydrogen peroxide. The yeast acts as a catalyst for this reaction.
The oxygen should be inflating the balloon. Let this reaction go on for a time.
Meanwhile, light your candle and then cover it with the jar. Watch what happens to the flame. Don’t get too close to the jar. If it is weak, it could crack.

Look at your balloon. It should be partially inflated. Pinch the open end of the balloon and carefully take it off the bottle making sure no oxygen escapes. Keep the end pinched closed.

What Happened? Oxygen and Fire Experiment
Oxygen is necessary for fire. When the jar was placed over the candle the first time, the flame went out when all the oxygen was consumed.
We created oxygen when the yeast was mixed with the hydrogen peroxide. Hydrogen peroxide is not a stable compound. It is always decomposing into water and oxygen. It is kept in dark bottles to slow this reaction.
Yeast acts as a catalyst to speed up the decomposition of hydrogen peroxide. This is how we are able to collect a balloon full of oxygen.
When the oxygen from the balloon was placed into the jar with the lit candle, you should have seen the flame grow brighter. This was caused by the increased amount of oxygen in the jar. Fire actually burns faster (and appears brighter) when the oxygen concentration increases.
In this Steel Wool and 9 Volt Battery experiment , you can also see how the presence of heat and oxygen causes a combustion reaction and fire.

Physical Science Lesson
You can find this oxygen and fire experiment in Module 2 of Apologia’s Physical Science, 2nd Edition, curriculum. This module is all about air, its components, and their relationships to life on Earth.
Two years ago, she studied Human Anatomy with Apologia. You can see some of our experiments and projects in our Science Saturday series.
I hold a master’s degree in child development and early education and am working on a post-baccalaureate in biology. I spent 15 years working for a biotechnology company developing IT systems in DNA testing laboratories across the US. I taught K4 in a private school, homeschooled my children, and have taught on the mission field in southern Asia. For 4 years, I served on our state’s FIRST Lego League tournament Board and served as the Judging Director. I own thehomeschoolscientist and also write a regular science column for Homeschooling Today Magazine. You’ll also find my writings on the CTCMath blog. Through this site, I have authored over 50 math and science resources.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Do you want to play with fire? This is a collection of science projects involving fire, including how to color fire, fire magic tricks, and interesting chemistry demonstrations involving fire and flames.

How to Make Pink Flames – Pink Fire Tutorial

Colored Fire Spray Bottles

How to Make Red Flames (Red Fire)

How to Make Blue Fire

How to Make Colored Fire Pinecones

How to Make Golden and Yellow Fire

Why Is Fire Hot? How Hot Is It?

How to Make Purple Fire

How to Make White Fire

IMAGES
COMMENTS
Please be careful when you will be performing these fire experiments at home or at school. All of these fire tricks can be extremely dangerous so again, please be careful. Always use safety glasses or face-shield, gloves, well-ventilated areas and adult supervision. Its good to have prepared fire extinguisher. Subscribe if you like ;-)
Fire is the result of combustion, which is when oxygen and a fuel is heated and reacts with each other. Fire - the flame itself - consists of fuel and oxygen molecules falling apart and emitting light and heat. To experiment with fire is a great way to learn about molecules, states of matter, chemical reactions, heat and energy.
Sep 10, 2022 · PLAY WITH FIRE | 10 SCIENCE EXPERIMENTS10_science_experiments #playwithfire #color_of_flames#experimentsSubscribe to our Fun Science & YouTube Channel HERE ...
10 Awesome Fire Tricks and Science Experiments you can do at home!SUBSCRIBE: http://bit.ly/2kcNkI21: TRAVELING FLAME - Blow out the fire on a candle and hold...
Exploding fire science experiments are wonderful features at live science museum shows. These experiments "wow" the crowd and help to show something about science, but they are not safe unless the building is set up correctly. This fire science experiment is not exploding, but is great for teaching kids about the science of fire. Materials Needed
Fire science fair projects and experiments: topics, ideas, resources, and sample projects by scientific field.
This experiment uses carbon dioxide created from the chemical reaction between baking soda and vinegar to put out a fire. How to Make the DIY Fire Extinguisher: For this experiment, you will need some small tea light candles, baking soda, vinegar, matches, a funnel and a bottle or pitcher with a lid.
You can find this oxygen and fire experiment in Module 2 of Apologia’s Physical Science, 2nd Edition, curriculum. This module is all about air, its components, and their relationships to life on Earth. Two years ago, she studied Human Anatomy with Apologia. You can see some of our experiments and projects in our Science Saturday series.
Dec 28, 2021 · This is a collection of science projects involving fire, including how to color fire, fire magic tricks, and interesting chemistry demonstrations involving fire and flames. Learn how to make pink flames using common, readily available chemicals.
Apr 9, 2024 · Instructions for Fire Experiments Three Different Ways to Explore Fire Fire Experiment #1. We sprayed water on a burning candle. The cool water turned to steam when it touches the flame thereby taking away the necessary heat that the fire needs to continuing burning. Findings: If a fire is hot then fight it by making it cool! Fire Experiment #2